Hox protein
From Proteopedia
| Line 10: | Line 10: | ||
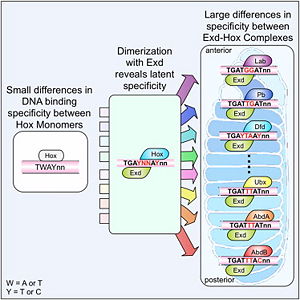
[[Image:Cell.jpg|thumb|right|300px|Figure 2: Latent specificity of Hox proteins <ref name="slattery">Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147(6):1270-82.</ref>.]] | [[Image:Cell.jpg|thumb|right|300px|Figure 2: Latent specificity of Hox proteins <ref name="slattery">Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147(6):1270-82.</ref>.]] | ||
| - | Hox proteins are transcription factors that play a key role in the embryonic development across species. Eight ''Drosophila'' Hox proteins are responsible for the development of different body segments of the fly, for example its antennae, wings, or legs. Hox proteins execute their distinct functions through binding to closely related but different in vivo binding sites. This page discusses the mechanisms through which Hox proteins recognize their DNA targets with very high binding specificity. <br/> | + | Hox proteins are transcription factors that play a key role in the '''embryonic development''' across species. Eight ''Drosophila'' Hox proteins are responsible for the development of different body segments of the fly, for example its antennae, wings, or legs. Hox proteins execute their distinct functions through binding to closely related but different in vivo binding sites. This page discusses the mechanisms through which Hox proteins recognize their DNA targets with very high binding specificity. <br/> |
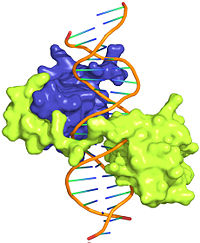
| - | The crystal structure of a Hox-DNA complex (Figure 1) shows for the Hox protein Sex combs reduced (Scr) that it binds its specific in vivo site with the help of a cofactor, Extradenticle (Exd). Hox proteins bind DNA as monomers but their biniding specificity of enhanced when the co-factor is present, a principle that is called 'latent specificity' (Figure 2). In | + | The crystal structure of a Hox-DNA complex (Figure 1) shows for the Hox protein Sex combs reduced (Scr) that it binds its specific in vivo site with the help of a cofactor, Extradenticle (Exd). Hox proteins bind DNA as monomers but their biniding specificity of enhanced when the co-factor is present, a principle that is called '''latent specificity''' (Figure 2). In ''Drosophila'' for instance, eight Hox proteins bind as heterodimer with their cofactor Exd to similar but distinct target sites. Hox proteins are expressed along the anterior-posterior axis of an embryo, thus determining the localization for the development of different body segments (Figure 2). This spatial order of expression from anterior to posterior is congruent with the location of the respective Hox binding sites at the chromosome, a fact knowm as '''collinearity'''. <br/> |
Revision as of 01:31, 3 July 2012
| This Sandbox is Reserved from 22 April 2012, through 31 August 2012 for use in the course "Protein DNA" taught by Remo_Rohs at the La Canada High School, USA. This reservation includes Sandbox Reserved 169 through Sandbox Reserved 170. |
To get started:
More help: Help:Editing |
Hox Proteins Specifically Recognize the Sequence-Dependent Shape of the Minor Groove
Biological Role of Hox Proteins
Hox proteins are transcription factors that play a key role in the embryonic development across species. Eight Drosophila Hox proteins are responsible for the development of different body segments of the fly, for example its antennae, wings, or legs. Hox proteins execute their distinct functions through binding to closely related but different in vivo binding sites. This page discusses the mechanisms through which Hox proteins recognize their DNA targets with very high binding specificity.
The crystal structure of a Hox-DNA complex (Figure 1) shows for the Hox protein Sex combs reduced (Scr) that it binds its specific in vivo site with the help of a cofactor, Extradenticle (Exd). Hox proteins bind DNA as monomers but their biniding specificity of enhanced when the co-factor is present, a principle that is called latent specificity (Figure 2). In Drosophila for instance, eight Hox proteins bind as heterodimer with their cofactor Exd to similar but distinct target sites. Hox proteins are expressed along the anterior-posterior axis of an embryo, thus determining the localization for the development of different body segments (Figure 2). This spatial order of expression from anterior to posterior is congruent with the location of the respective Hox binding sites at the chromosome, a fact knowm as collinearity.
|
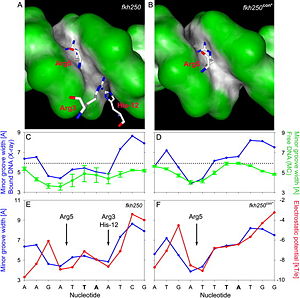
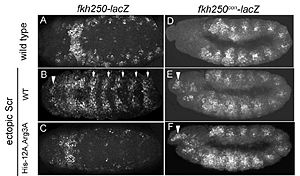
References
- ↑ 1.0 1.1 1.2 Joshi R, Passner JM, Rohs R, Jain R, Sosinsky A, Crickmore MA, Jacob V, Aggarwal AK, Honig B, Mann RS. Functional specificity of a Hox protein mediated by the recognition of minor groove structure. Cell. 2007;131(3):530-43.
- ↑ 2.0 2.1 Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147(6):1270-82.
Proteopedia Page Contributors and Editors (what is this?)
Remo Rohs, Eric Martz, Michal Harel, Joel L. Sussman, Skyler Saleebyan, Julia Tam, Bailey Holmes, Sharon Kim, Alexander Berchansky, Iris Dror, Ana Carolina Dantas Machado, Masha Karelina, Keziah Kim, Jaime Prilusky, Angel Herraez
