Carnitine acyltransferases are a large family of enzymes that play a main role in cellular energy metabolism, i.e. fatty acid oxidation. These enzymes catalyze the reversible exchange of acyl groups (which derive from fatty acids) between coenzyme A and carnitine.
Carnitine acyltransferases include three different classes of enzymes which are known as carnitine acetyltransferases (CrATs), carnitine octanoyltransferases (COTs) and carnitine palmityltransferases (CPTs). "The three classes differ in their acyl group specificity as well as their localization." [1]. Nevertheless functional conservation between the carnitine acyltransferases can be observed. That's why the focus of this entry is on the structure of carnitine acetyltransferase as a representitive of carnitine acyltransferases. Determining the structure and thus the molecular basis for fatty acid transfer is needed for drug development. Being major enzymes in fatty acid oxidation carnitine acyltransferases are viewed as promising targets which can be used to develop successful therapeutics against type 2 diabetes, obesity and other human diseases.
Biological function
- Differences within carnitine acyltransferase family members
Carnitine acyltransferases carry out pivotal biological functions. All members of carnitine acyltransferases catalyze the same reversible reaction:the exchange of acyl groups between carnitine and coenzyme A (CoA). This is in accord with the fact, that the catalytic domains of all the carnitine acyltransferases are well conserved.
However the three known classes of carnitine acyltransferases show differences in their acyl group specificity and subcellular localization.
Carnitine acetyltransferase (CrATs) prefere short chain fatty acids as substrates and are localized in the mitochondrial matrix, the endoplasmic reticulum, and the peroxisome.
Carnitine octanoyltransferases (COTs) show medium chain fatty acid substrate preference and are mainly found in peroxisomes.
Carnitine palmitoyltransferase (CPTs) show long chain fatty acid substrate preference and are found in the outer mitochondrial
membrane and the mitochondrial matrix.[1]
- The role of carnitine acyltransferases in fatty acid oxidation
The most important biological function of carnitine acyltransferases is the transport of fatty acids for β-oxidation[2].Fatty acids are oxidized for energy production in the mitochondrial matrix by a process called β- oxidation. The major site of fatty acid accumulation, however, is the cytoplasm of the cells. Hence, in order to provide energy, fatty acids have to be transported from the cytoplasm across the inner mitochondrial membrane into the mitochondrial matrix. The carnitine shuttle, a transport chain that consists of three enzymatic reactions, helps fatty acids to pass the mitochondrial membrane. Carnitine acyltransferases (CrATs, COTs, CPTs) are part of the carnitine shuttle.
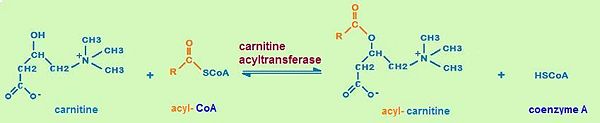
second step of the carnitine shuttle
The first step of the carnitine shuttle is the activation of fatty acids. They are transformed into the activated form (= acyl CoA) by the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A. The second step of the carnitine shuttle is catalyzed by carnitine acyltransferases. These enzymes (especially CPT) facilitate the transport of fatty acids by conjugating them to carnitine. In this reaction an acyl group is transferred from the sulfur atom of CoA to the hydroxyl group of carnitine. The product is acyl- carnitine. The third step of the carnitine shuttle is also catalyzed by carnitine acyltransferases (especially CPT) in the mitochondrial matrix. In this reaction the acyl group of acyl- carnitine ester is transferred back to CoA to form acyl- CoA. [3]
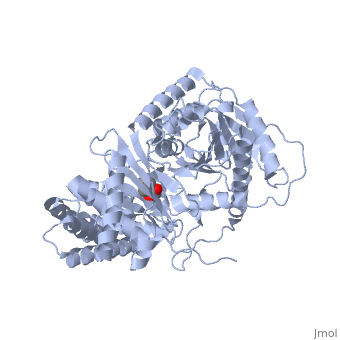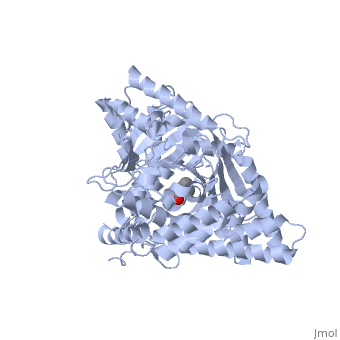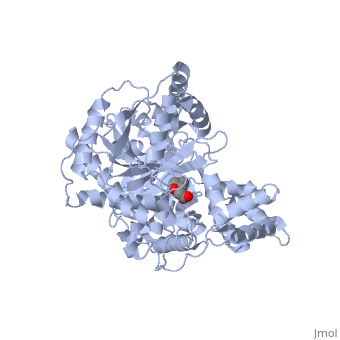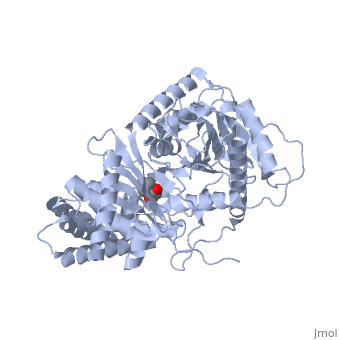
Structure of Carnitine acetyltransferase
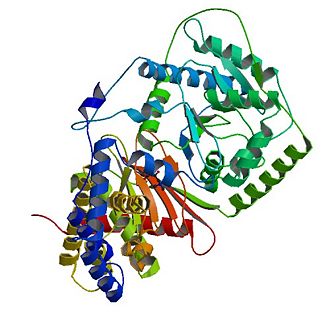
The structure of CrAT contains 16 β strands (β1–β16) and 20 α helices (α1–α20) and can be divided into two domains, a C domain and a N domain.[4] The shown 3D structure shows two molecules of carnitine acetyltransferases and thus two active sites as well as two carnitine binding sites.
The core of the 2 domains is a 6-stranded mixed β-sheet (β10-β13, β15, β16 in the C domain), with 3 α-helices covering one of its faces (α6, α7, α12 in the N domain). The other face of the β-sheet in the N domain is covered by additional helices, whereas the other face of the β-sheet in the C domain is covered by the N domain.
The of CrAT is located at the interface between the N and C domains. Biochemical and mutagenesis studies have permitted to identify a of carnitine acyltransferases. It can be reached from two openings of the tunnel on the surface of the structure. One of these openings is used for binding carnitine, while the other is used for binding CoA. Hence, the two substrates of the enzyme bind at opposite sides of the catalytic residue.
The carnitine substrate has to be positioned in a way that the proton of its hydroxyl group can interact with the nitrogen N3 of the catalytic residue histidine 343. Carnitine binding in the right position is made possible by electrostatic interactions and the formation of hydrogen bondings between the carboxylate group of carnitine and active site residues. Residues that form the carnitine binding site can be found in the C domain and in the N domain.
The main are tyrosine452, serine454, and threonine465. They possess side chain hydroxyls which can interact with the carboxylic oxygen atoms of carnitine. One of the carboxylic oxygen atoms is also hydrogen-bonded to a water molecule [2] Electrostatic interactions are formed by the carboxylate group of carnitine with the side chain guanidinium group of an arginine residue.
The exact role of the trimethylammonium group during carnitine binding hasn’t been fully revealed yet. Carnitine is rather required for catalysis than for binding. Even though the trimethylammonium group has a positive charge on its nitrogen it is not surrounded by negatively charged residues which could balance it. Instead, the trimethylammonium group is situated in a rather hydrophobic environment.
There are only slight conformational changes in the enzyme upon carnitine binding.“The only significant conformational difference in the active site between the free enzyme and the carnitine complex is in the side chain of serine454, which adopts a different rotamer to have better hydrogen-bonding interactions with the carboxylate of carnitine.” [2]</StructureSection>
Catalytic Mechanism of Carnitine Acyltransferases
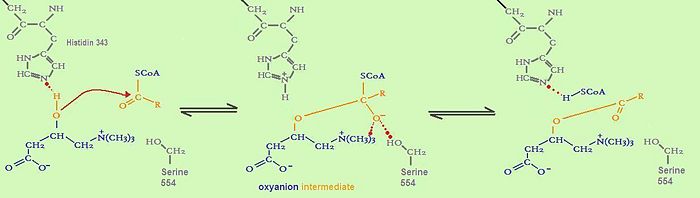
catalytic mechanism of fatty acid transfer
It is assumed that the whole family of carnitine acyltransferases share the same catalytic mechanism, because certain residues in the catalytic side (histidine343, serine554) are conserved throughout the family.
Histidine 343 is probably the most important residue in catalysis. First, it induces optimal substrate binding by forming a hydrogen bond between its side chain and the hydrogen atom of the substrate’s reactive group. As soon as all substrates attained the right position, the catalytic histidine residue is ready to extract a proton from either the hydroxyl group of carnitine or the thiol group of CoA. The catalytic histidine residue can be considered as a general base in catalysis.Which proton is extracted depends on the direction of the reaction. Acyl- carnitine is formed by extracting a proton from carnitine, whereas acyl-CoA is formed by extracting a proton from CoA.
The extraction of a hydrogen atom leads to the development of a tetrahedral oxyanion intermediate. This oxyanion is stabilized by the side chain hydroxyl of serine 554 through hydrogen bonding as well as by the positive charge on the trimethylammonium group of carnitine. As the positive charge of the carnitine substrate is necessary for the carnitine acyltransferase mediated reaction to happen, this catalysis can be described as substrate-assisted catalysis.[4]
Regulation
One of the most common regulation systems of carnitine acyltransferases involves inhibition by malonyl-CoA, an intermediate in the synthesis of fatty acids. Malonyl-CoA inhibits long-chain carnitine acyltransferase activity by all three enzymes at similar concentrations in the physiological range. Moreover, the mitochondrial (CAT) and peroxisomal (COT) enzymes can also be regulated through mRNA transcription by a number of shared factors. Although the microsomal enzyme is less well studied, there does, indeed, appear to be a pattern of coordinate regulation for this system.
Carnitine acetyltransferase deficiency and diseases
Mutation and dysregulation of CPTs are linked to serious human diseases. Recessive mutations of CPT-I and CPT-IICPT-I and CPT-II are crucial for the beta-oxidation of long-chain fatty acids in the mitochondria by enabling their transport across the mitochondrial membrane. can produce hypoketonemia and hypoglycemia in patients, while CPT-II deficiency is the most common cause of abnormal lipid metabolism in skeletal muscle.Single-point mutations as well as insertions/deletions in the CPT genes can produce the clinical phenotype. The hypoglycemia observed in patients with reduced CPT-I activity suggests that antagonists of CPT-Is may be able to lower blood glucose levels. A covalent CPT-I inhibitor, etomoxir, can lower blood glucose levels in diabetic animals and humans, showing that such inhibitors may be efficacious for the treatment of type 2 diabetes.
3D structures of carnitine acetyltransferase
1ndb – mCAT – mouse
1t7n – mCAT (mutant)
1ndf – mCAT + carnitine
1t7o – mCAT (mutant) + carnitine
2h3u – mCAT + carnitine + CoA
1t7q – mCAT (mutant) + carnitine + CoA
2h3w – mCAT (mutant) + hexanoylcarnitine + CoA
2h3p – mCAT + Carnitine + acetyl-CoA
1ndi – mCAT + CoA
1s5o – CAT + carnitine - human
References
- ↑ 1.0 1.1 Donghai Wu‡, Lakshmanan Govindasamy§, Wei Lian‡, Yunrong Gu‡, Thomas Kukar‡,Mavis Agbandje-McKenna§, and Robert McKenna§¶.Structure of Human Carnitine Acetyltransferase.Published, JBC Papers in Press, January 31, 2003 DOI 10.1074/jbc.M21235620
- ↑ 2.0 2.1 2.2 Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003 Jan 10;112(1):113-22. PMID:12526798
- ↑ Lehninger Principles of Biochemistry |ISBN-10: 071677108X | ISBN-13: 978-0716771081 | Publication Date: February 1, 2008 | Edition: 5th
- ↑ 4.0 4.1 Jogl G, Hsiao YS, Tong L. Structure and function of carnitine acyltransferases. Ann N Y Acad Sci. 2004 Nov;1033:17-29. PMID:15591000 doi:10.1196/annals.1320.002