We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Fragment-Based Drug Discovery
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
= Drug Design: SAR by NMR = | = Drug Design: SAR by NMR = | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='500' side='right' caption='Bcl-xl in complex with ABT-737 (PDB entry [[2yxj]])' scene='Sandbox_reserved_394/Bcl-xl_abt-737_complex/2'> |
Traditionally, new drugs are developed by either making small changes to existing drugs or by individually testing thousands of compounds. Both of these methods require many hours of laborious chemical synthesis. However, new techniques that capitalize on the advances of modern technology are being applied in the drug industry to develop new drugs which decrease the cost and time required to discover and develop new drugs. Nuclear magnetic resonance (NMR) and x-ray crystallography can be used to analyze compounds in order to create three-dimensional images for detailed, visual analysis of those compounds. Applying these 3-D structures to the drug design process involves using either structure-based drug design (SBDD) or ligand-based drug design (LBDD). | Traditionally, new drugs are developed by either making small changes to existing drugs or by individually testing thousands of compounds. Both of these methods require many hours of laborious chemical synthesis. However, new techniques that capitalize on the advances of modern technology are being applied in the drug industry to develop new drugs which decrease the cost and time required to discover and develop new drugs. Nuclear magnetic resonance (NMR) and x-ray crystallography can be used to analyze compounds in order to create three-dimensional images for detailed, visual analysis of those compounds. Applying these 3-D structures to the drug design process involves using either structure-based drug design (SBDD) or ligand-based drug design (LBDD). | ||
{| class="wikitable collapsible collapsed" | {| class="wikitable collapsible collapsed" | ||
| Line 17: | Line 17: | ||
---- | ---- | ||
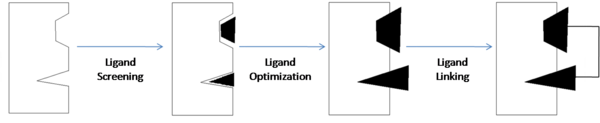
Structure-activity relationship (SAR) by (NMR) is one tool that is commonly used to design and develop new drugs. This is the process "in which small organic molecules that bind to proximal subsites of a protein are identified, optimized, and linked together to produce high-affinity ligands."<ref name="Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.">Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.</ref> | Structure-activity relationship (SAR) by (NMR) is one tool that is commonly used to design and develop new drugs. This is the process "in which small organic molecules that bind to proximal subsites of a protein are identified, optimized, and linked together to produce high-affinity ligands."<ref name="Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.">Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.</ref> | ||
| + | [[Image:SAR by NMR Illustrated.png | thumb | center | 600px | SAR by NMR]] | ||
==== ABT-737 ==== | ==== ABT-737 ==== | ||
| Line 38: | Line 39: | ||
Once the components responsible for binding are identified, they can be modified, as in the case of compound 1 where the carboxylic acid was substituted with an acyl sulfonamide, and then they are linked together to create a compound with optimal binding affinity. | Once the components responsible for binding are identified, they can be modified, as in the case of compound 1 where the carboxylic acid was substituted with an acyl sulfonamide, and then they are linked together to create a compound with optimal binding affinity. | ||
</StructureSection> | </StructureSection> | ||
| - | |||
= References = | = References = | ||
<references/> | <references/> | ||
Revision as of 03:01, 30 October 2012
Drug Design: SAR by NMR
| |||||||||||
References
- ↑ Pandit D. LIGAND-BASED DRUG DESIGN: I. CONFORMATIONAL STUDIES OF GBR 12909 ANALOGS AS COCAINE ANTAGONISTS; II. 3D-QSAR STUDIES OF SALVINORIN A ANALOGS AS εΑΡΡΑ OPIOID AGONISTS. http://archives.njit.edu/vol01/etd/2000s/2007/njit-etd2007-051/njit-etd2007-051.pdf
- ↑ Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.
- ↑ Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579