We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Josie N. Harmon/Sandbox Tutorial
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
== Xanthine Oxidase Biochemistry Tutorial == | == Xanthine Oxidase Biochemistry Tutorial == | ||
| - | The | + | The purpose of this tutorial is to explain the mechanism of the metabolic enzyme xanthine oxidoreductase. |
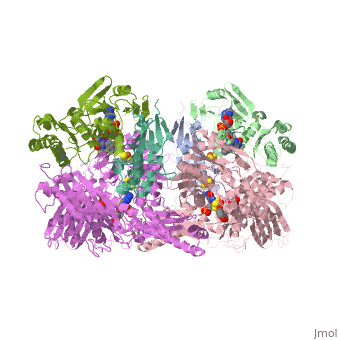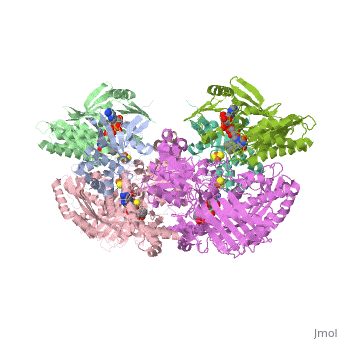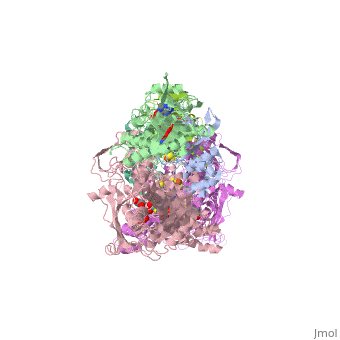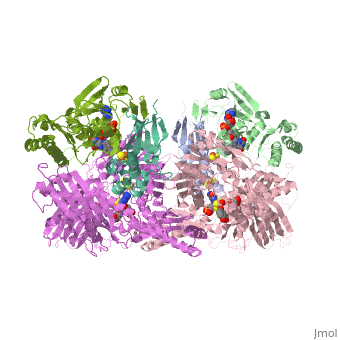
<StructureSection load='1fiq' size='350' side='right' caption='Crystal Structure of Xanthine Oxidase from Bovine Milk (PDB entry [[1fiq]])' scene=''> | <StructureSection load='1fiq' size='350' side='right' caption='Crystal Structure of Xanthine Oxidase from Bovine Milk (PDB entry [[1fiq]])' scene=''> | ||
== Xanthine Oxidoreductase == | == Xanthine Oxidoreductase == | ||
| - | Xanthine oxidoreductase is actualy considered to | + | Xanthine oxidoreductase is actualy considered to exist as two enzymes in one, one being xanthine oxidase and the other existing as xanthine dehydrogenase. When the enzyme was initially purified by scientists two different forms of the enzyme were found, where one uses nicotinamide adenine dinucleotide (NAD) and the other uses oxygen. The two forms were believed to exist as two different enzymes and were thus given two different names; however, upon further investigation into the amino acid sequence of the enzymes it was determined that the enzymes were actually the same. The two forms of the enzyme can differ in two ways: |
| - | 1. | + | 1. Within the oxidoreductase enzyme there are multiple disulfide bridges. In the case that these bridges are left untouched the enzyme will then take on the characteristics to act as an oxidase, but in the event that these bridges are restricted the enzyme then acts as a dehydrogenase. |
| - | 2. The oxidoreductase enzyme | + | 2. The oxidoreductase enzyme is permanently altered by proteases so that it will constantly utilized in the oxidase form. |
| + | |||
| + | == Mechanism of Action == | ||
| + | Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. In eukaryotes xanthine oxidase exist as a homodimer with each monomer containing four redox-active sites. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, Fe2S2, clusters, and FAD. | ||
== Electron Extraction == | == Electron Extraction == | ||
| - | |||
One side of the xanthine oxidoreductase enzyme consists of an active site that includes a molybdenum atom which binds to a purine substrate and adds a hydroxyl group. During this process electrons are extracted and funneled from the active site through a string of iron-sulfur clusters to the opposing side of the enzyme. The opposing side then transfers the electrons to NAD or oxygen depending on the dehydrogenase or oxidase nature of the enzyme. One of the final steps in the electron transfer funnels electrons to a FAD group. The dehydrogenase form of the enzyme transfers these electrons to NAD, while the oxidase form blocks NAD through a loop of protein that covers the FAD molecule allowing smaller oxygen molecules to accept the electrons. | One side of the xanthine oxidoreductase enzyme consists of an active site that includes a molybdenum atom which binds to a purine substrate and adds a hydroxyl group. During this process electrons are extracted and funneled from the active site through a string of iron-sulfur clusters to the opposing side of the enzyme. The opposing side then transfers the electrons to NAD or oxygen depending on the dehydrogenase or oxidase nature of the enzyme. One of the final steps in the electron transfer funnels electrons to a FAD group. The dehydrogenase form of the enzyme transfers these electrons to NAD, while the oxidase form blocks NAD through a loop of protein that covers the FAD molecule allowing smaller oxygen molecules to accept the electrons. | ||
| - | |||
| - | == Mechanism of Action == | ||
| - | Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. In eukaryotes xanthine oxidases exist as homodimers with each monomer containing four redox-active sites. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, Fe2S2, clusters, and FAD. | ||
== Metabolism == | == Metabolism == | ||
Revision as of 16:24, 12 November 2012
Xanthine Oxidase Biochemistry Tutorial
The purpose of this tutorial is to explain the mechanism of the metabolic enzyme xanthine oxidoreductase.
| |||||||||||