RA Mediated T-reg Differentiation
From Proteopedia
| Line 7: | Line 7: | ||
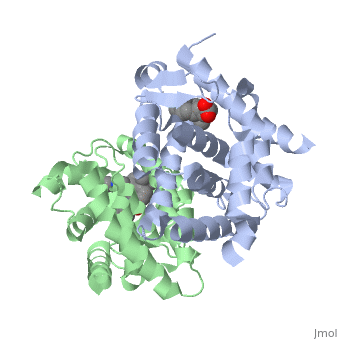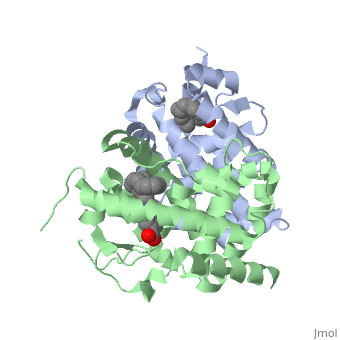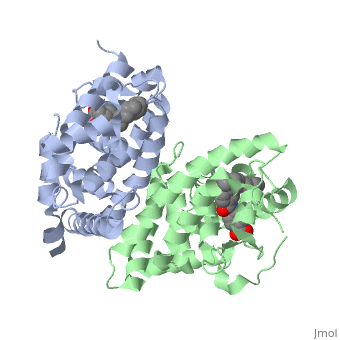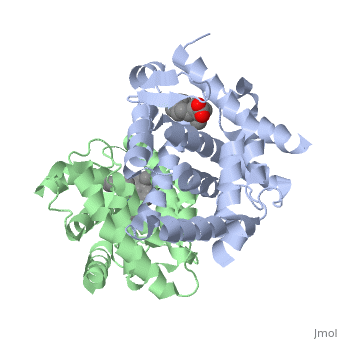
=== RARα-RXRα Heterodimer === | === RARα-RXRα Heterodimer === | ||
| - | When these two proteins form a heterodimer, the overall structure of the larger dimer is comparable to that of an RXRα homodimer, likely due to the many similarities these two molecules share. RARα and RXRα rely on residues from the H7, H8, H9, H10, L8-9, and L9-10 domains of both molecules to form the <scene name='RA_Mediated_T-reg_Differentiaition/Dimerization_interface/1'>heterodimer interface</scene>. The sequence identity between the two molecules on the dimer interface is 0.33, demonstrating that 33% of the interacting residues are homologous between the different proteins. | + | When these two proteins form a heterodimer, the overall structure of the larger dimer is comparable to that of an RXRα homodimer, likely due to the many similarities these two molecules share. RARα and RXRα rely on residues from the H7, H8, H9, H10, L8-9, and L9-10 domains of both molecules to form the <scene name='RA_Mediated_T-reg_Differentiaition/Dimerization_interface/1'>heterodimer interface</scene>. The sequence identity between the two molecules on the dimer interface is 0.33, demonstrating that 33% of the interacting residues are homologous between the different proteins. |
| Line 21: | Line 21: | ||
Negatively charged residues: E357, D384, E395, E399, E406, and E439 (red); | Negatively charged residues: E357, D384, E395, E399, E406, and E439 (red); | ||
Positively charged residues: R353, K361, R398, K410, K422, R426, R431, and K436 (blue); | Positively charged residues: R353, K361, R398, K410, K422, R426, R431, and K436 (blue); | ||
| - | Hydrophilic residues: S432 (green). | + | Hydrophilic residues: S432 (green). <ref> PMID: 10882070 </ref> |
| + | |||
=== RARα-RXRα Ligand Interactions === | === RARα-RXRα Ligand Interactions === | ||
| - | Upon binding of the ligand ATRA in the cytoplasm, RARα and RXRα form a heterodimer that alters the DNA binding domains of both subunits in a manner that allows them to bind to specific DNA sequences. The two proteins have 29% identity in their LBD. | + | Upon binding of the ligand ATRA in the cytoplasm, RARα and RXRα form a heterodimer that alters the DNA binding domains of both subunits in a manner that allows them to bind to specific DNA sequences. The two proteins have 29% identity in their <scene name='RA_Mediated_T-reg_Differentiaition/Ligand_binding_pockets/1'>LBD</scene>. |
For the ligand used in RARα crystallization, BMS614, 21 primarily hydrophobic residues form the <scene name='RA_Mediated_T-reg_Differentiaition/Rar-ligand_binding_pocket/1'>RAR-alpha ligand binding pocket</scene>. BMS614 is not the natural ligand for this molecule, but acts as an more stable agonist for crystallization. The largest difference between BMS614 and ATRA upon binding to the pocket are at Ile 412, where BMS614 pushes much closer to the amino acid than ATRA does. | For the ligand used in RARα crystallization, BMS614, 21 primarily hydrophobic residues form the <scene name='RA_Mediated_T-reg_Differentiaition/Rar-ligand_binding_pocket/1'>RAR-alpha ligand binding pocket</scene>. BMS614 is not the natural ligand for this molecule, but acts as an more stable agonist for crystallization. The largest difference between BMS614 and ATRA upon binding to the pocket are at Ile 412, where BMS614 pushes much closer to the amino acid than ATRA does. | ||
| - | The major differences between RARα, RARβ and RARγ are present in this area: | + | The <scene name='RA_Mediated_T-reg_Differentiaition/Rar-ligand_binding_pocket2/1'>major differences</scene> between RARα, RARβ and RARγ are present in this area: |
Residue 270: α:Ile β:Ile γ:Met; Residue 232: α:Ser β:Ala γ:Ala; Residue 395: α:Val β:Val γ:Ala | Residue 270: α:Ile β:Ile γ:Met; Residue 232: α:Ser β:Ala γ:Ala; Residue 395: α:Val β:Val γ:Ala | ||
Revision as of 08:55, 14 November 2012
Introduction
T-regulatory cells (T-regs) are a small subset of CD4+ T-cells that exhibit strong down regulation of immune system activity in their local environment. They are distinguished from other CD4+ T-cells by the expression of FOXP3, a gene regulator. [1] The exact mechanisms used by T-regs to down regulate the immune system has not yet been clearly elucidated. These cells have been shown to differentiate from CD4+ T-helper cells upon activation and exposure to the following cytokines: tumor growth factor β (TGF-β), Interleukin-2 (IL-2) and retinoic acid (RA). [2] Both TGF-β and IL-2 are used in other immune system differentiation, however, RA has been shown to bias T-cells to the T-reg phenotype. [3] When acting upon T-reg cells, RA acts as the ligand for the Retinoic Acid Receptor-α (RARα) / Retinoid X Receptor-α (RXRα) heterodimer. This heterodimer is of the nuclear receptor family, and each chain consists of the same three part structure: a Ligand binding domain (LBD), a DNA binding domain (DBD), and a hinge region connecting the two binding domains. [4]
| |||||||||||
| |||||||||||
Biological Significance
References
- ↑ Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009 May;123(5):977-83; quiz 984-5. PMID:19410687 doi:10.1016/j.jaci.2009.03.030
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999 May;64(5):310-9. PMID:10406480
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
Proteopedia Page Contributors and Editors (what is this?)
William Bailey, Alexander Berchansky, Michal Harel, Jaime Prilusky