Herceptin - Mechanism of Action
From Proteopedia
| Line 3: | Line 3: | ||
== '''Basics''' == | == '''Basics''' == | ||
=== Introduction === | === Introduction === | ||
| - | Breast cancer is the most common type of cancer found among women except for skin cancers <ref name=" | + | Breast cancer is the most common type of cancer found among women except for skin cancers<ref name="fourteen">"What Are the Key Statistics about Breast Cancer?" Breast Cancer. Cancer.org, 31 Oct. 2012. Web. Nov. 2012. <http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics>.</ref>. Although it is rarely seen in men, one in eight women will be diagnosed with breast cancer within their lifetime. Patients exhibiting an over-expression in Human Epidermal Growth Factor Receptor 2 (HER2) account for 25% of all breast cancer <ref name="two"> Cho, Hyun-Soo, Karen Mason, Kasra X. Ramyar, Ann Marie Stanley, Sandra B. Gabelli, Dan W. Denney, Jr., and Daniel J. Leahy. "Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab." Letters to Nature 421 (2003): 756-60. PubMed.gov. Web. Oct. 2012. </ref><ref name="three"> "HER2 Dimerization: A Key Component of Oncogenic Signaling in HER2 Breast Cancer." HER2+ Breast Cancer. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/hdis/her2-dimerization/index.html>. </ref>. HER2+ patients often experience a more aggressive cancer resulting in more metastasized tumors <ref name="three"/>. The statistics show a poor prognosis for HER2+ patients with a 5-year survival rate of 68%. Herceptin (also known as trastuzumab) was approved by the FDA in September of 1998 for HER2+ patients and has been shown to be an effective tool in the battle against breast cancer <ref name="four">"Herceptin Development Timeline." Genentech: Medicines. Genentech, n.d. Web. Nov. 2012. <http://www.gene.com/gene/products/information/oncology/herceptin/timeline.html>.</ref>. |
=== HER2 === | === HER2 === | ||
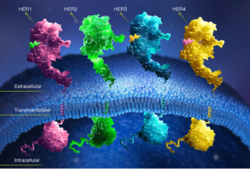
| - | HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4)<ref name="five"> Bazley, L. A., and W. J. Gullick. "The Epidermal Growth Factor Receptor Family." Endocrine-Related Cancer. Society for Endocrinology and European Society of Endocrinology, 2005. Web. Oct. 2012. <http://erc.endocrinology-journals.org/content/12/Supplement_1/S17.full>. </ref><ref name="six">Satyanarayanajois, Seetharama, Stephanie Villalba, Liu Jianchao, and Go Mei Lin. "Design, Synthesis, and Docking Studies of Peptidomimetics." Chem. Biol. Drug Des. 74.3 (2009): 246-57. National Institute of Health. Web. Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2866155/pdf/nihms-190276.pdf>.</ref>. These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation <ref name="seven">Banappagari, Sashikanth, Sharon Ronald, and Seetharama Satyanarayanajois. "Structure-activity Relationship of Conformationally Constrained." Medchemcomm. 2.8 (2011): 752-59. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3163471/pdf/nihms308284.pdf>.</ref | + | HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4)<ref name="five"> Bazley, L. A., and W. J. Gullick. "The Epidermal Growth Factor Receptor Family." Endocrine-Related Cancer. Society for Endocrinology and European Society of Endocrinology, 2005. Web. Oct. 2012. <http://erc.endocrinology-journals.org/content/12/Supplement_1/S17.full>. </ref><ref name="six">Satyanarayanajois, Seetharama, Stephanie Villalba, Liu Jianchao, and Go Mei Lin. "Design, Synthesis, and Docking Studies of Peptidomimetics." Chem. Biol. Drug Des. 74.3 (2009): 246-57. National Institute of Health. Web. Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2866155/pdf/nihms-190276.pdf>.</ref>. These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation<ref name="two"/><ref name="seven">Banappagari, Sashikanth, Sharon Ronald, and Seetharama Satyanarayanajois. "Structure-activity Relationship of Conformationally Constrained." Medchemcomm. 2.8 (2011): 752-59. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3163471/pdf/nihms308284.pdf>.</ref>. [[Image:New Resource 1.jpg|thumb|right|300 px|(Figure 2) HER family<ref name="three"/>]] This family is known as the ErbB family, being that these proteins are encoded by the ERBB genes, and is also known as the HER family. The HER family are plasma membrane-bound and contain an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain <ref name="three"/>. |
These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors) <ref name="two"/>. Over 11 different ligands for the epidermal growth factor receptors have been identified <ref name="five"/>. After binding with these ligands the HER family is able to homodimerize or heterodimerize with one another <ref name="eight">"HER Pathways Are of Critical Importance in Cancer." HER Receptors Overview. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/her/overview/index.html>.</ref>. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway. | These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors) <ref name="two"/>. Over 11 different ligands for the epidermal growth factor receptors have been identified <ref name="five"/>. After binding with these ligands the HER family is able to homodimerize or heterodimerize with one another <ref name="eight">"HER Pathways Are of Critical Importance in Cancer." HER Receptors Overview. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/her/overview/index.html>.</ref>. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway. | ||
HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner <ref name="two"/><ref name="eight"/>. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor. <ref name="eight"/> | HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner <ref name="two"/><ref name="eight"/>. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor. <ref name="eight"/> | ||
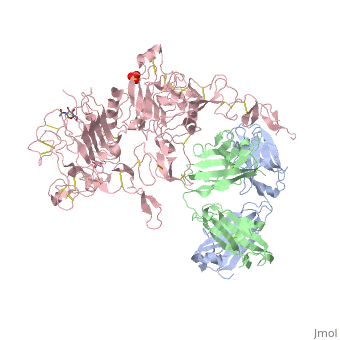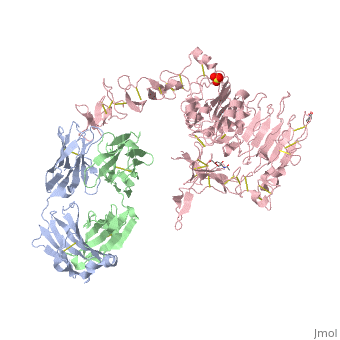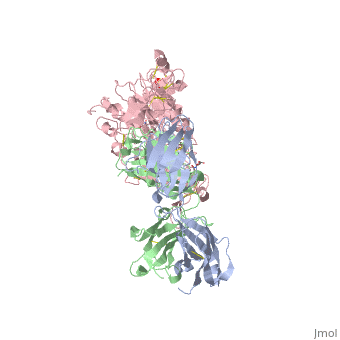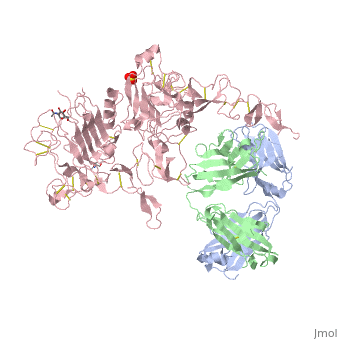
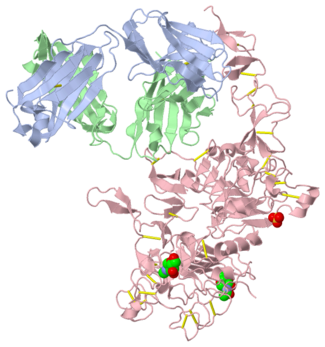
| - | [[Image:HerceptinFab.jpg|thumb|left|250 px|(Figure 3) Herceptin]] | + | [[Image:HerceptinFab.jpg|thumb|left|250 px|(Figure 3) Herceptin <ref name="thirteen">Ryzhkov, Andrew. "Trastuzumab." Wikipedia. Wikimedia Foundation, 11 Aug. 2012. Web. 09 Nov. 2012. <http://en.wikipedia.org/wiki/Trastuzumab>.</ref>]] |
=== Herceptin === | === Herceptin === | ||
| Line 19: | Line 19: | ||
== '''Structures and Interactions''' == | == '''Structures and Interactions''' == | ||
=== HER2 === | === HER2 === | ||
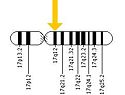
| - | [[Image:Human Chromosome 17.jpg|thumb|right|125 px|(Figure 4) Human Chromosome 17]] | + | [[Image:Human Chromosome 17.jpg|thumb|right|125 px|(Figure 4) Human Chromosome 17<ref name="ten"/>]] |
''ERBB2'' is located on the long arm of human chromosome 17 (17q12). This gene codes for the protein HER2 and is known as a proto-oncogene due to its ability to become an oncogene from an over-expression <ref name="ten">"ERBB2." Genetics Home Reference. U.S. National Library of Medicine, 5 Nov. 2012. Web. 09 Nov. 2012. <http://ghr.nlm.nih.gov/gene/ERBB2>.</ref>. | ''ERBB2'' is located on the long arm of human chromosome 17 (17q12). This gene codes for the protein HER2 and is known as a proto-oncogene due to its ability to become an oncogene from an over-expression <ref name="ten">"ERBB2." Genetics Home Reference. U.S. National Library of Medicine, 5 Nov. 2012. Web. 09 Nov. 2012. <http://ghr.nlm.nih.gov/gene/ERBB2>.</ref>. | ||
| Line 28: | Line 28: | ||
*<scene name='Sandbox_reserved_Herceptin/Sub-domain_iv/3'>subdomain IV</scene> | *<scene name='Sandbox_reserved_Herceptin/Sub-domain_iv/3'>subdomain IV</scene> | ||
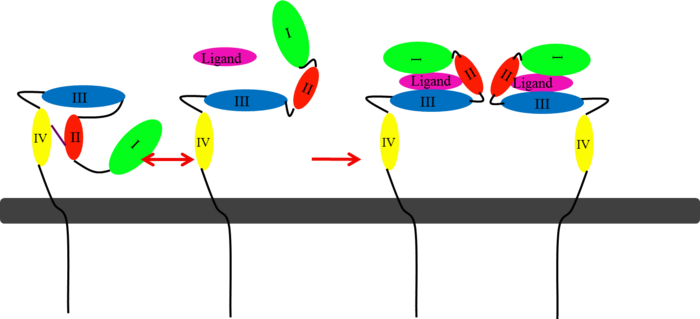
| - | In EGFR, HER3, and HER4 sub-domains I and III remain in a “closed” conformation until a ligand binds <ref name="two"/><ref name="eleven">Lammerts Van Bueren, Jeroen, Wim K. Bleeker, Annika Bra¨ Nnstro¨m, Anne Von Euler, Magnus Jansson, Matthias Peipp, Tanja Schneider-Merck, Thomas Valerius, Jan G. J. Van De Winkel, and Paul W. Parren. "The Antibody Zalutumumab Inhibits Epidermal Growth." PNAS 105.16 (2008): 6109-114. Web. Nov. 2012. <http://www.pnas.org/content/105/16/6109/F1.expansion.html>.</ref>. Subdomain II remains hidden making contact with sub-domain IV creating a "tether" (See figure 6) <ref name="eleven"/>. The tether between sub-domain II and IV can be temporarily broken in order for the receptor to become more available for a ligand to bind. Although unsure, evidence suggests that the ligand binds to subdomain I and/or subdomain III; also, there’s some evidence of ligand-binding to subdomain IV <ref name=" | + | In EGFR, HER3, and HER4 sub-domains I and III remain in a “closed” conformation until a ligand binds <ref name="two"/><ref name="eleven">Lammerts Van Bueren, Jeroen, Wim K. Bleeker, Annika Bra¨ Nnstro¨m, Anne Von Euler, Magnus Jansson, Matthias Peipp, Tanja Schneider-Merck, Thomas Valerius, Jan G. J. Van De Winkel, and Paul W. Parren. "The Antibody Zalutumumab Inhibits Epidermal Growth." PNAS 105.16 (2008): 6109-114. Web. Nov. 2012. <http://www.pnas.org/content/105/16/6109/F1.expansion.html>.</ref>. Subdomain II remains hidden making contact with sub-domain IV creating a "tether" (See figure 6) <ref name="eleven"/>. The tether between sub-domain II and IV can be temporarily broken in order for the receptor to become more available for a ligand to bind. Although unsure, evidence suggests that the ligand binds to subdomain I and/or subdomain III; also, there’s some evidence of ligand-binding to subdomain IV<ref name="two"/><ref name="one"> Saxon, Marian L., and David C. Lee. "Mutagenesis Reveals a Role for Epidermal Growth Factor Receptor Extracellular Subdomain IV in Ligand Binding." The Journal of Biological Chemistry 274.40 (1999): 28356-8362. PubMed.gov. Web. Oct. 2012. <http://www.jbc.org/content/274/40/28356.long>. </ref>. Once ligand-binding is initiated, the receptor experiences a conformational change allowing subdomains I and III to become closer together resulting in the “open” conformation <ref name="two"/>. This open conformation exposes subdomain II which allows for dimerization between receptors. Although some evidence suggests that sub-domain IV is necessary for ligand-binding, stabilizing the extracellular domain, and locking the receptor in an open conformation, the exact function is still unknown. Once the dimerization between the two receptors takes place, a cross-phosphorylation reaction between the two tyrosine kinases occurs following the activation of certain cell signaling pathways <ref name="eight"/>. These pathways include: |
*mitogen-activated protein kinase (MAPK) | *mitogen-activated protein kinase (MAPK) | ||
*phosphoinositide 3-kinase (PI3K/Akt) | *phosphoinositide 3-kinase (PI3K/Akt) | ||
| Line 35: | Line 35: | ||
*protein kinase C (PKC) | *protein kinase C (PKC) | ||
| - | [[Image:EGFRs.png|thumb|left|250 px|(Figure 5) HER family structures]] | + | [[Image:EGFRs.png|thumb|left|250 px|(Figure 5) HER family structures<ref name="three"/>]] |
HER2 structure differs in that subdomains I and III are in constant contact resulting in a permanent open conformation <ref name="two"/><ref name="three"/>. Subdomains I and III in HER2 are stabilized by core hydrophobic residues surrounded by hydrophilic contacts facilitating the stabilization of this interaction <ref name="two"/>. This fixed conformation allows the receptor to act in a ligand-independent manner and permits the access of subdomain II for dimerization. Perhaps this may explain why a high-affinity ligand for HER2 has not yet been recognized. The absence of subdomain II and IV contact can be explained by the mutations of Gly 563 and His 565 found in the other members of the HER family to Pro and Phe respectively. Since subdomain II is always available for dimerization it is free to form heterodimers with any of the other epidermal growth factor receptors. Asp 285 is the only residue in subdomain II not conserved between EGFR and HER2. Leu replaces Asp 285 in HER2 and can explain why HER2 forms homodimers to a lesser extent. Once dimerized, HER2 activates the MAPK pathway, which in turn initiates cell proliferation, migration, differentiation, and angiogenesis <ref name="three"/>. | HER2 structure differs in that subdomains I and III are in constant contact resulting in a permanent open conformation <ref name="two"/><ref name="three"/>. Subdomains I and III in HER2 are stabilized by core hydrophobic residues surrounded by hydrophilic contacts facilitating the stabilization of this interaction <ref name="two"/>. This fixed conformation allows the receptor to act in a ligand-independent manner and permits the access of subdomain II for dimerization. Perhaps this may explain why a high-affinity ligand for HER2 has not yet been recognized. The absence of subdomain II and IV contact can be explained by the mutations of Gly 563 and His 565 found in the other members of the HER family to Pro and Phe respectively. Since subdomain II is always available for dimerization it is free to form heterodimers with any of the other epidermal growth factor receptors. Asp 285 is the only residue in subdomain II not conserved between EGFR and HER2. Leu replaces Asp 285 in HER2 and can explain why HER2 forms homodimers to a lesser extent. Once dimerized, HER2 activates the MAPK pathway, which in turn initiates cell proliferation, migration, differentiation, and angiogenesis <ref name="three"/>. | ||
| Line 61: | Line 61: | ||
One limitation with this therapy is that it does not prevent HER2 from dimerizing with HER3 <ref name="three"/>. This makes it possible for the activation of the PI3K pathway which increases the ability of the cell to survive. Further therapies targeting the prevention of HER2 to dimerize with other members of the HER family will be necessary for future investigational studies. | One limitation with this therapy is that it does not prevent HER2 from dimerizing with HER3 <ref name="three"/>. This makes it possible for the activation of the PI3K pathway which increases the ability of the cell to survive. Further therapies targeting the prevention of HER2 to dimerize with other members of the HER family will be necessary for future investigational studies. | ||
| - | </StructureSection>[[Image:EGFRs Mechanism.png|700px|left|thumb| <span style="font-size:1.2em;">(Figure 6) This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family.</span>]] | + | </StructureSection>[[Image:EGFRs Mechanism.png|700px|left|thumb| <span style="font-size:1.2em;">(Figure 6) This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family.</span><ref name="eleven"/>]] |
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 14:31, 14 November 2012
Basics
Introduction
Breast cancer is the most common type of cancer found among women except for skin cancers[1]. Although it is rarely seen in men, one in eight women will be diagnosed with breast cancer within their lifetime. Patients exhibiting an over-expression in Human Epidermal Growth Factor Receptor 2 (HER2) account for 25% of all breast cancer [2][3]. HER2+ patients often experience a more aggressive cancer resulting in more metastasized tumors [3]. The statistics show a poor prognosis for HER2+ patients with a 5-year survival rate of 68%. Herceptin (also known as trastuzumab) was approved by the FDA in September of 1998 for HER2+ patients and has been shown to be an effective tool in the battle against breast cancer [4].
HER2
HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4)[5][6]. These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation[2][7].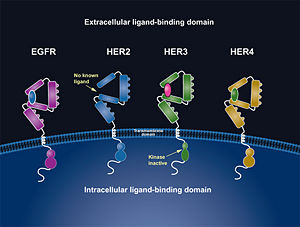
These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors) [2]. Over 11 different ligands for the epidermal growth factor receptors have been identified [5]. After binding with these ligands the HER family is able to homodimerize or heterodimerize with one another [8]. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway.
HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner [2][8]. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor. [8]
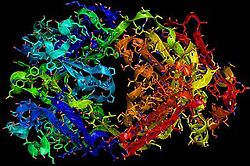
Herceptin
Herceptin, generic trastuzumab, is a monoclonal antibody [10]. Herceptin is an effective treatment for breast cancer for the reason that it binds to the extracellular domain of HER2 and, by multiple mechanisms of action, can prevent cell proliferation as well as target these HER2+ cells for destruction by the immune system [3][10].
| |||||||||||
References
- ↑ "What Are the Key Statistics about Breast Cancer?" Breast Cancer. Cancer.org, 31 Oct. 2012. Web. Nov. 2012. <http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics>.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Cho, Hyun-Soo, Karen Mason, Kasra X. Ramyar, Ann Marie Stanley, Sandra B. Gabelli, Dan W. Denney, Jr., and Daniel J. Leahy. "Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab." Letters to Nature 421 (2003): 756-60. PubMed.gov. Web. Oct. 2012.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 "HER2 Dimerization: A Key Component of Oncogenic Signaling in HER2 Breast Cancer." HER2+ Breast Cancer. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/hdis/her2-dimerization/index.html>.
- ↑ "Herceptin Development Timeline." Genentech: Medicines. Genentech, n.d. Web. Nov. 2012. <http://www.gene.com/gene/products/information/oncology/herceptin/timeline.html>.
- ↑ 5.0 5.1 Bazley, L. A., and W. J. Gullick. "The Epidermal Growth Factor Receptor Family." Endocrine-Related Cancer. Society for Endocrinology and European Society of Endocrinology, 2005. Web. Oct. 2012. <http://erc.endocrinology-journals.org/content/12/Supplement_1/S17.full>.
- ↑ 6.0 6.1 Satyanarayanajois, Seetharama, Stephanie Villalba, Liu Jianchao, and Go Mei Lin. "Design, Synthesis, and Docking Studies of Peptidomimetics." Chem. Biol. Drug Des. 74.3 (2009): 246-57. National Institute of Health. Web. Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2866155/pdf/nihms-190276.pdf>.
- ↑ Banappagari, Sashikanth, Sharon Ronald, and Seetharama Satyanarayanajois. "Structure-activity Relationship of Conformationally Constrained." Medchemcomm. 2.8 (2011): 752-59. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3163471/pdf/nihms308284.pdf>.
- ↑ 8.0 8.1 8.2 8.3 "HER Pathways Are of Critical Importance in Cancer." HER Receptors Overview. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/her/overview/index.html>.
- ↑ Ryzhkov, Andrew. "Trastuzumab." Wikipedia. Wikimedia Foundation, 11 Aug. 2012. Web. 09 Nov. 2012. <http://en.wikipedia.org/wiki/Trastuzumab>.
- ↑ 10.0 10.1 Jiang, Beihai, Wenbin Liu, Hong Qu, Lin Meng, Shumei Song, Tao Ouyang, and Chengchao Shou. "A Novel Peptide Isolated from a Phage Display Peptide Library with." The Journal of Biological Chemistry 280.6 (2005): 4656-662. The Journal of Biological Chemistry. Web. Oct. 2012. <http://www.jbc.org/content/280/6/4656.full.pdf+html>.
- ↑ 11.0 11.1 "ERBB2." Genetics Home Reference. U.S. National Library of Medicine, 5 Nov. 2012. Web. 09 Nov. 2012. <http://ghr.nlm.nih.gov/gene/ERBB2>.
- ↑ 12.0 12.1 12.2 Lammerts Van Bueren, Jeroen, Wim K. Bleeker, Annika Bra¨ Nnstro¨m, Anne Von Euler, Magnus Jansson, Matthias Peipp, Tanja Schneider-Merck, Thomas Valerius, Jan G. J. Van De Winkel, and Paul W. Parren. "The Antibody Zalutumumab Inhibits Epidermal Growth." PNAS 105.16 (2008): 6109-114. Web. Nov. 2012. <http://www.pnas.org/content/105/16/6109/F1.expansion.html>.
- ↑ Saxon, Marian L., and David C. Lee. "Mutagenesis Reveals a Role for Epidermal Growth Factor Receptor Extracellular Subdomain IV in Ligand Binding." The Journal of Biological Chemistry 274.40 (1999): 28356-8362. PubMed.gov. Web. Oct. 2012. <http://www.jbc.org/content/274/40/28356.long>.
- ↑ 14.0 14.1 14.2 Gajria, Devika, and Sarat Chandarlapaty. HER2-amplified Breast Cancer: Mechanisms of Trastuzumab 11.2 (2011): 263-75. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092522/pdf/nihms289124.pdf>.