Sandbox Reserved 647
From Proteopedia
| Line 9: | Line 9: | ||
== Introduction == | == Introduction == | ||
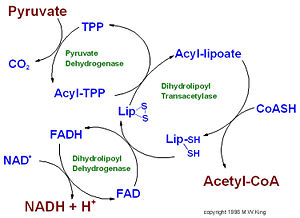
| - | '''Pyruvate dehydrogenase''' (E1) along with two other enzymes make up the pyruvate dehydrogenase multienzyme complex. Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3) are the other two main components of the complex. Along with the associated prosthetic groups, Thiamine pyrophosphate (TPP), Lipoamide, and Flavin Adenine Dinucleotide (FAD) respective to each enzyme. These enzymes work together to synthesize acetyl Co-A by the irreversible oxidative decarboxylation of pyruvate before entering the Kreb's cycle. This catalytic reaction also produces NADH and carbon dioxide as side products. The pyruvate dehydrogenase complex acts to link glycolysis to the citric acid cycle. The pyruvate dehydrogenase complex is localized in the matrix space of the mitochondria and is responsible for regulating the flow of energy in cells by determining when pyruvate should be converted into acetyl Co-A ore neutralized to lactic acid to allow continued glycolysis. | + | '''Pyruvate dehydrogenase''' (E1) along with two other enzymes make up the pyruvate dehydrogenase multienzyme complex. Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3) are the other two main components of the complex. Along with the associated prosthetic groups, Thiamine pyrophosphate (TPP), Lipoamide, and Flavin Adenine Dinucleotide (FAD) respective to each enzyme. These enzymes work together to synthesize acetyl Co-A by the irreversible oxidative decarboxylation of pyruvate before entering the Kreb's cycle. This catalytic reaction also produces NADH and carbon dioxide as side products. The pyruvate dehydrogenase complex acts to link glycolysis to the citric acid cycle. The pyruvate dehydrogenase complex is localized in the matrix space of the mitochondria in eukaryotes and the cytosol of bacteria. This complex is responsible for regulating the flow of energy in cells by determining when pyruvate should be converted into acetyl Co-A ore neutralized to lactic acid to allow continued glycolysis. |
[[Image:Pyruvate _ oxidation.jpg | 300 px | thumb | left]] | [[Image:Pyruvate _ oxidation.jpg | 300 px | thumb | left]] | ||
Revision as of 22:48, 14 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
IntroductionPyruvate dehydrogenase (E1) along with two other enzymes make up the pyruvate dehydrogenase multienzyme complex. Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3) are the other two main components of the complex. Along with the associated prosthetic groups, Thiamine pyrophosphate (TPP), Lipoamide, and Flavin Adenine Dinucleotide (FAD) respective to each enzyme. These enzymes work together to synthesize acetyl Co-A by the irreversible oxidative decarboxylation of pyruvate before entering the Kreb's cycle. This catalytic reaction also produces NADH and carbon dioxide as side products. The pyruvate dehydrogenase complex acts to link glycolysis to the citric acid cycle. The pyruvate dehydrogenase complex is localized in the matrix space of the mitochondria in eukaryotes and the cytosol of bacteria. This complex is responsible for regulating the flow of energy in cells by determining when pyruvate should be converted into acetyl Co-A ore neutralized to lactic acid to allow continued glycolysis.
StructurePyruvate dehydrogenase consists of a combination of beta strands and alpha helices.
Functionthis is the site of between three residues of pyruvate dehydrogenase.
RegulationThe pyruvate dehydrogenase complex is regulated through reversible phosphorylation. The phosphorylation events occur on three serine residues at sites that are located on the pyruvate dehydrogenase (E1) alpha subunit. Once all three of the serine residues are phosphorylated the entire complex is inhibited, thus preventing the synthesis of Acetyl-CoA. The phosphorylation events are catalyzed by the enzyme pyruvate dehydrogenase kinase (PDK). Pyruvate dehydrogenase kinase exists in four know isoforms PDK1-PDK4. These kinases are distributed differently in various tissues. Activation of these kinases are also regulated by different factors. PDK1 is activated when there is a low concentration of oxygen available. PDK2 is activated when there is a high amount of Acetyl-CoA and NADH already present. PDK3 is activated when there is a high abundance of ATP already accessible for use. PDK4 is activated when the cell is suffering from starvation and nutrient deprivation. Dephosphorylation of the serine residues to restore the activity of the pyruvate dehydrogenase complex is catalyzed by the enzyme pyruvate dehydrogenase phosphatase (PDP). This enzyme has two known isoforms PDP1 and PDP2. PDP1 is prevalent in skeletal muscles and PDP2 is prevalent in the liver and adipocytes. |