We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sean Swale/Human Thrombin Inhibitor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='3utu' size='500' frame='false' align='right' |caption='Human Thrombin' scene=''> | |
| - | < | + | |
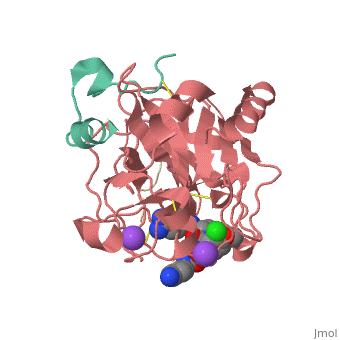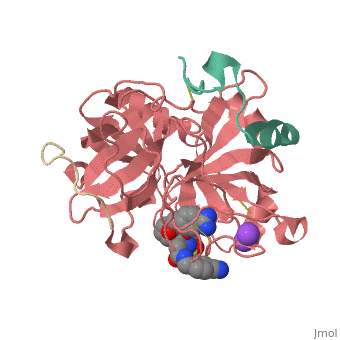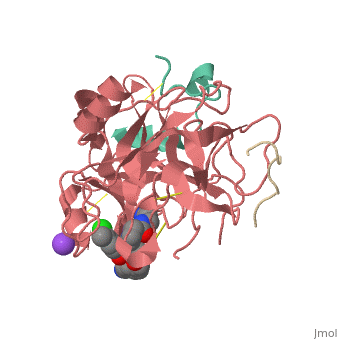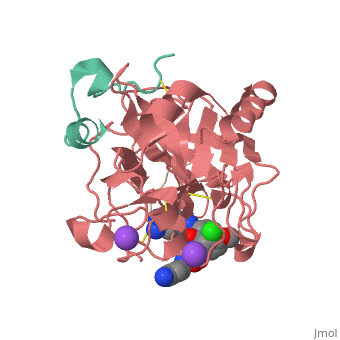
<scene name='Sean_Swale/Human_Thrombin_Inhibitor/Active_site/1'>Total active site</scene> | <scene name='Sean_Swale/Human_Thrombin_Inhibitor/Active_site/1'>Total active site</scene> | ||
===Reasons to develop another Thrombin Inhibitor=== | ===Reasons to develop another Thrombin Inhibitor=== | ||
Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area<ref>January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25 </ref>. However, when fibrin has accumulated at an injury site, sometimes it will break away and occlude a blood vessel which can cause stroke or heart attack. In order to prevent venous thrombosis, blood clots, after surgery or during dialysis when blood is in contact with artificial devices, Heparin is normally administered. Unfortunately Heparin is neutralized by plasma proteins in the blood, is less efficient for the inhibition of clot bound thrombin, and can trigger an enormous immune response. Antithrombotic drugs like Hirudin will be given if Heparin is not able to be tolerated or if the person receives regular dialysis. Hirudin administration require large doses to maintain proper inhibition of Thrombin which heavily taxes the body. Chemists have been working on designer drugs to increase selectivity and potency. One compound which has shown great selectivity and potency in vitro is inhibitor 65.<ref> DOI: 10.1002/cmdc.201200292</ref> | Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area<ref>January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25 </ref>. However, when fibrin has accumulated at an injury site, sometimes it will break away and occlude a blood vessel which can cause stroke or heart attack. In order to prevent venous thrombosis, blood clots, after surgery or during dialysis when blood is in contact with artificial devices, Heparin is normally administered. Unfortunately Heparin is neutralized by plasma proteins in the blood, is less efficient for the inhibition of clot bound thrombin, and can trigger an enormous immune response. Antithrombotic drugs like Hirudin will be given if Heparin is not able to be tolerated or if the person receives regular dialysis. Hirudin administration require large doses to maintain proper inhibition of Thrombin which heavily taxes the body. Chemists have been working on designer drugs to increase selectivity and potency. One compound which has shown great selectivity and potency in vitro is inhibitor 65.<ref> DOI: 10.1002/cmdc.201200292</ref> | ||
===Phe-Pro-p-amidinobenzylamine=== | ===Phe-Pro-p-amidinobenzylamine=== | ||
| - | An early experiment to find a better thrombin inhibitor yielded a structure that was able to bond to the P1 and P2 sites of Thrombin effectively. In 1996 Lilly research laboratories created D-Phe-Pro-p-amidinobenzylamine by making improvements on the previous structure D-Phe-Pro-Agmatine. The new compound they created bonded to thrombin 130x more than it did to trypsin (also a serine protease), and it was highly selective of thrombin over fibrinolytic enzymes | + | An early experiment to find a better thrombin inhibitor yielded a structure that was able to bond to the P1 and P2 sites of Thrombin effectively. In 1996 Lilly research laboratories created D-Phe-Pro-p-amidinobenzylamine by making improvements on the previous structure D-Phe-Pro-Agmatine. The new compound they created bonded to thrombin 130x more than it did to trypsin (also a serine protease), and it was highly selective of thrombin over fibrinolytic enzymes which break down fibrin clots. The inhibitor bound with Thrombin at rates 26,000x greater than with plasmin,<ref> http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68</ref>, 170,000x greater than with t-Pa, and 400,000x greater than with urokinase. |
| + | |||
| + | |||
| + | Its selectivity was gained by binding its nitrogens on the benzamidine group by hydrogen bonding to Asp 189, and by fitting its benzamidine benzene into a hydrophobic pocket with Ser 214-Glu 217 and Asp 189-Glu 192 on the other side. The proline residue is in another hydrophobic pocket made up of His 57, Tyr 60A, Leu 99, and Trp 60D. The rest of this structure is unimportant on review of inhibitor 65 because only the amidinobenzylamine which occupies R1 and the proline which occupies the R2 sit are the same residues on inhibitor 65.<ref> M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428 </ref> | ||
===P3 and P4 of Inhibitor 65=== | ===P3 and P4 of Inhibitor 65=== | ||
| Line 11: | Line 13: | ||
</StructureSection> | </StructureSection> | ||
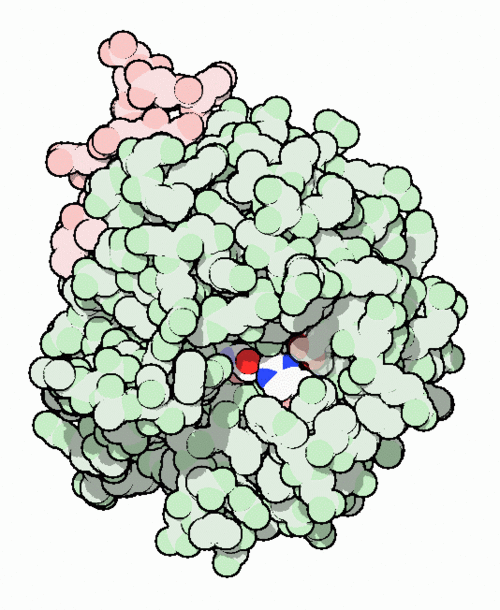
This is the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/Thrombin_active_site/2'>active site</scene> of thrombin | This is the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/Thrombin_active_site/2'>active site</scene> of thrombin | ||
| - | + | ||
| - | [[Image:1ppb-transparent.gif|right| | + | [[Image:1ppb-transparent.gif|right|500px]] |
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 20:54, 15 November 2012
| |||||||||||
This is the of thrombin
References
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428