We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sean Swale/Human Thrombin Inhibitor
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
===P3 and P4 of Inhibitor 65=== | ===P3 and P4 of Inhibitor 65=== | ||
[[Image:Inhibitor 65.png|center|400px|frame|<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | [[Image:Inhibitor 65.png|center|400px|frame|<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | ||
| - | Inhibitor 65 additionally has a <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4/1'>P4</scene> sulfonyl group with a methoxy group at the end of the residue. The residue sits in a hydrophobic <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_binding_pocket/1'>pocket</scene>. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.The <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_sulfonyl/1'>sulfonyl</scene> group holds its own VanderWaal forces. Additionally, the chlorine points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P3_binding_site/6'>residue</scene>, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to a water molecule which then connects to Glu 192, and the hydrogens of Gly 219 and Gly 216. | + | Inhibitor 65 additionally has a <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4/1'>P4</scene> sulfonyl group with a methoxy group at the end of the residue. The residue sits in a hydrophobic <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_binding_pocket/1'>pocket</scene>. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.The <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_sulfonyl/1'>sulfonyl</scene> group holds its own VanderWaal forces. Additionally, the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_chlorine/1'>chlorine</scene> points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P3_binding_site/6'>residue</scene>, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to a water molecule which then connects to Glu 192, and the hydrogens of Gly 219 and Gly 216. |
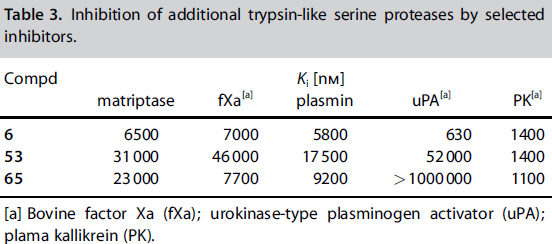
[[Image:Thrombin1.gif|left|frame|300px|a) Stereo view of the complete inhibitor structure provided with its electron density in the active site of thrombin. Thrombin is shown with its solvent- accessible surface. b) The P4 sulfonyl residue is located in the aryl binding pocket. The chlorine atom points to the carbonyl oxygen of Asn 98 and accommodates a position above the indole moiety of Trp 215. The methoxy group perfectly fills the upper niche of the S3/4 pocket. c) The P3 side chain is directed into the solvent, where its amide NH binds via a bridging water molecule to Glu 192, Gly 216 and Gly 219.<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | [[Image:Thrombin1.gif|left|frame|300px|a) Stereo view of the complete inhibitor structure provided with its electron density in the active site of thrombin. Thrombin is shown with its solvent- accessible surface. b) The P4 sulfonyl residue is located in the aryl binding pocket. The chlorine atom points to the carbonyl oxygen of Asn 98 and accommodates a position above the indole moiety of Trp 215. The methoxy group perfectly fills the upper niche of the S3/4 pocket. c) The P3 side chain is directed into the solvent, where its amide NH binds via a bridging water molecule to Glu 192, Gly 216 and Gly 219.<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | ||
Revision as of 23:54, 15 November 2012
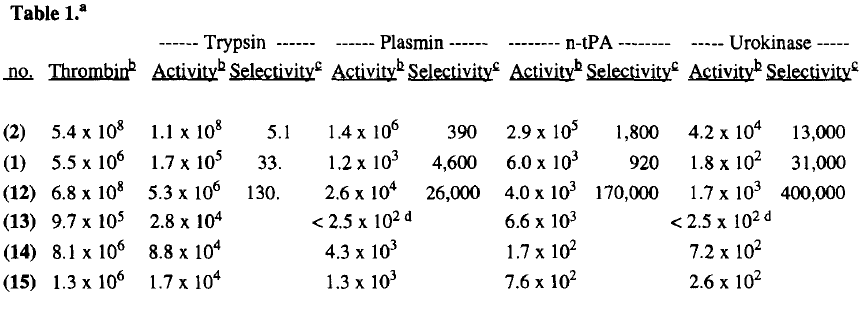
| |||||||||||
This is the of thrombin
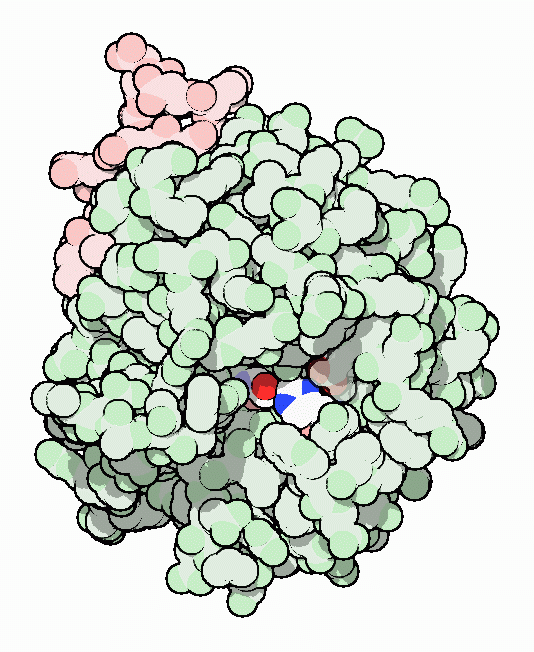
Thrombin with serine residue visible.[8]
References
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25