Sandbox Reserved 390
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
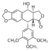
In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex<ref>PMID: 21778401</ref>. This structure provided the first observation of a TOP2 ternary cleavage complex <scene name='Sandbox_Reserved_390/Top/22'>stabilized</scene> by an anticancer drug. | In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex<ref>PMID: 21778401</ref>. This structure provided the first observation of a TOP2 ternary cleavage complex <scene name='Sandbox_Reserved_390/Top/22'>stabilized</scene> by an anticancer drug. | ||
| - | The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/ | + | The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/23'>drugs</scene>. This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents. |
==COMPOUND ACTIVE SITE== | ==COMPOUND ACTIVE SITE== | ||
Revision as of 22:28, 19 November 2012
Human topoisomerase IIbeta in complex with DNA and etoposide
| |||||||||||
References
- ↑ Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID:21778401 doi:10.1126/science.1204117
- ↑ Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. DOI: 10.1093/nar/gkl057
- ↑ Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011 Jul 22;333(6041):459-62. PMID:21778401 doi:10.1126/science.1204117