Sandbox Reserved 649
From Proteopedia
(→'''Mechanism''') |
|||
| Line 2: | Line 2: | ||
{{Sandbox_Reserved_Robert_B_Rose_2}} | {{Sandbox_Reserved_Robert_B_Rose_2}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| + | |||
| + | |||
| + | = '''Argininosuccinate Lyase''' = | ||
| + | |||
| + | |||
| + | == '''Structure''' == | ||
== '''Mechanism''' == | == '''Mechanism''' == | ||
| - | + | ||
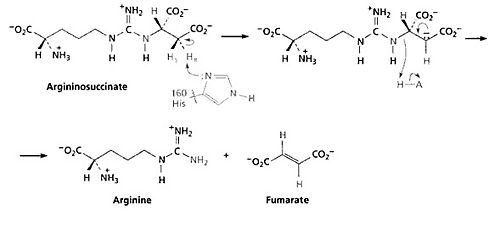
The third step of the urea cycle is catalyzed by argininosuccinate lyase. The products of this reaction are arginine and fumarate. The fumarate product is an important link between the urea cycle and the citric acid cycle. | The third step of the urea cycle is catalyzed by argininosuccinate lyase. The products of this reaction are arginine and fumarate. The fumarate product is an important link between the urea cycle and the citric acid cycle. | ||
| + | |||
| + | [[Image:Argininosuccinate.jpg |thumb|500 px|center]] | ||
The base initiates the reaction by deprotonating the carbon adjacent to the arginine, or leaving group. The process occurs by an E1cB mechanism with loss of the pro-R hydrogen (the hydrogen that will geive the R configuration of a molecule after it is abstracted) and with anti-stereochemistry. The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from mostly alky halides through a carbanion intermediate given specified reaction condition and specified substrates. There are some evidences have shown that Histidine 162 or Threonine 161 of ASL is responsible for the proton abstraction of the carbon, either directly or indirectly.<ref>The organic chemistry of biological pathways: McMurry, John, and Begley, Tadhg. Frank Vella. Article first published online: 3 NOV 2006.[http://books.google.com/books?id=slpol42h5D4C&pg=PA228&lpg=PA228&dq=The+organic+chemistry+of+biological+pathways+urea+cycle&source=bl&ots=YSpz3sf3cr&sig=QEdQS5DtSP02KqdRJP5S8E-OO2M&hl=en&sa=X&ei=F_WqUK-GAo689gSBq4CQBQ&ved=0CDAQ6AEwAA#v=onepage&q=The%20organic%20chemistry%20of%20biological%20pathways%20urea%20cycle&f=false]</ref> | The base initiates the reaction by deprotonating the carbon adjacent to the arginine, or leaving group. The process occurs by an E1cB mechanism with loss of the pro-R hydrogen (the hydrogen that will geive the R configuration of a molecule after it is abstracted) and with anti-stereochemistry. The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from mostly alky halides through a carbanion intermediate given specified reaction condition and specified substrates. There are some evidences have shown that Histidine 162 or Threonine 161 of ASL is responsible for the proton abstraction of the carbon, either directly or indirectly.<ref>The organic chemistry of biological pathways: McMurry, John, and Begley, Tadhg. Frank Vella. Article first published online: 3 NOV 2006.[http://books.google.com/books?id=slpol42h5D4C&pg=PA228&lpg=PA228&dq=The+organic+chemistry+of+biological+pathways+urea+cycle&source=bl&ots=YSpz3sf3cr&sig=QEdQS5DtSP02KqdRJP5S8E-OO2M&hl=en&sa=X&ei=F_WqUK-GAo689gSBq4CQBQ&ved=0CDAQ6AEwAA#v=onepage&q=The%20organic%20chemistry%20of%20biological%20pathways%20urea%20cycle&f=false]</ref> | ||
| Line 14: | Line 22: | ||
| - | '''Arginine Deficiency''' | + | === '''Arginine Deficiency''' === |
Argininosuccinate lyase deficiency is a urea cycle disorder which can present in the neonatal period or later in the childhood; this is a rare, autosomal recessive disorder. Argininosuccinate lyase is an intermediate enzyme in the urea synthesis pathway and its function is imperative to the continuation of the cycle. As result of this enzyme defect patients have increased ammonia, argininosuccinate, and citrulline accumulates in the blood. Ammonia builds to toxic levels, resulting in hyperammonnemia and may affect the nervous and eventually damage the liver. There is biochemical evidence that shows rises in ammonia can inhibit glutaminase and therefore limit the rate of synthesis of neurotransmitters such as glutamate, which could lead to the developmental delay in argininosuccinic aciduria patients.<ref>Ficicioglu C, Mandell R, Shih VE (November 2009). "Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening". Mol. Genet. Metab. 98 (3): 273–7.</ref><ref>Jack, JJB (1982). "Actions of ammonia on the central nervous system". Journal of Inherited Metabolic Disease 5 (S2): 104</ref> | Argininosuccinate lyase deficiency is a urea cycle disorder which can present in the neonatal period or later in the childhood; this is a rare, autosomal recessive disorder. Argininosuccinate lyase is an intermediate enzyme in the urea synthesis pathway and its function is imperative to the continuation of the cycle. As result of this enzyme defect patients have increased ammonia, argininosuccinate, and citrulline accumulates in the blood. Ammonia builds to toxic levels, resulting in hyperammonnemia and may affect the nervous and eventually damage the liver. There is biochemical evidence that shows rises in ammonia can inhibit glutaminase and therefore limit the rate of synthesis of neurotransmitters such as glutamate, which could lead to the developmental delay in argininosuccinic aciduria patients.<ref>Ficicioglu C, Mandell R, Shih VE (November 2009). "Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening". Mol. Genet. Metab. 98 (3): 273–7.</ref><ref>Jack, JJB (1982). "Actions of ammonia on the central nervous system". Journal of Inherited Metabolic Disease 5 (S2): 104</ref> | ||
| Line 22: | Line 30: | ||
ASL deficiency is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffectedand not a carrier. | ASL deficiency is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffectedand not a carrier. | ||
| - | '''Symptoms''' | + | ==== '''Symptoms''' ==== |
| - | + | '''Neonatal onset presents in the first 2-3 days of life''' | |
vomiting | vomiting | ||
| Line 38: | Line 46: | ||
coma worsen | coma worsen | ||
| - | + | '''Late onset''' | |
developmental delay | developmental delay | ||
| Line 48: | Line 56: | ||
skin and hair abnormalities | skin and hair abnormalities | ||
| - | '''Treatments''' | + | ==== '''Treatments''' ==== |
1)Treatment consists of a low protein diet, arginine supplementation to help complete the urea cycle, ammonia scavenging drugs in some cases and supplement carnitine if the patients have a secondary deficiency. | 1)Treatment consists of a low protein diet, arginine supplementation to help complete the urea cycle, ammonia scavenging drugs in some cases and supplement carnitine if the patients have a secondary deficiency. | ||
Revision as of 08:07, 20 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Argininosuccinate LyaseStructureMechanismThe third step of the urea cycle is catalyzed by argininosuccinate lyase. The products of this reaction are arginine and fumarate. The fumarate product is an important link between the urea cycle and the citric acid cycle. The base initiates the reaction by deprotonating the carbon adjacent to the arginine, or leaving group. The process occurs by an E1cB mechanism with loss of the pro-R hydrogen (the hydrogen that will geive the R configuration of a molecule after it is abstracted) and with anti-stereochemistry. The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from mostly alky halides through a carbanion intermediate given specified reaction condition and specified substrates. There are some evidences have shown that Histidine 162 or Threonine 161 of ASL is responsible for the proton abstraction of the carbon, either directly or indirectly.[1] ImplicationsArginine DeficiencyArgininosuccinate lyase deficiency is a urea cycle disorder which can present in the neonatal period or later in the childhood; this is a rare, autosomal recessive disorder. Argininosuccinate lyase is an intermediate enzyme in the urea synthesis pathway and its function is imperative to the continuation of the cycle. As result of this enzyme defect patients have increased ammonia, argininosuccinate, and citrulline accumulates in the blood. Ammonia builds to toxic levels, resulting in hyperammonnemia and may affect the nervous and eventually damage the liver. There is biochemical evidence that shows rises in ammonia can inhibit glutaminase and therefore limit the rate of synthesis of neurotransmitters such as glutamate, which could lead to the developmental delay in argininosuccinic aciduria patients.[2][3] Genetic Factors ASL deficiency is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffectedand not a carrier. SymptomsNeonatal onset presents in the first 2-3 days of life vomiting lethargy respiratory alkalosis hypothermia seizure coma worsen Late onset developmental delay intellectual disability seizures skin and hair abnormalities Treatments1)Treatment consists of a low protein diet, arginine supplementation to help complete the urea cycle, ammonia scavenging drugs in some cases and supplement carnitine if the patients have a secondary deficiency. 2)Liver transplant offers a partial correction of the enzyme deficiency and improved metabolic status. References
|