Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
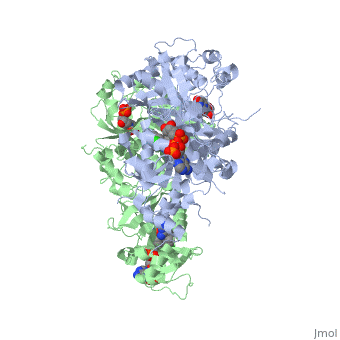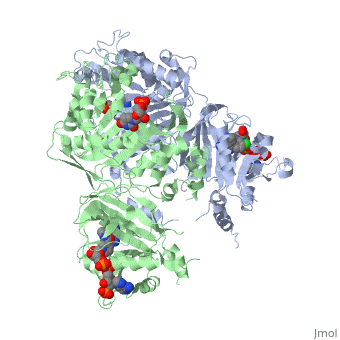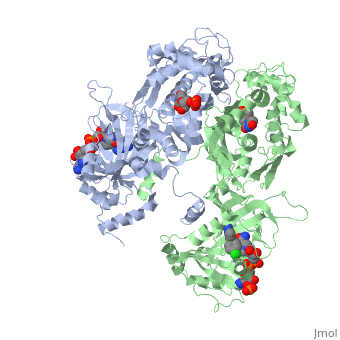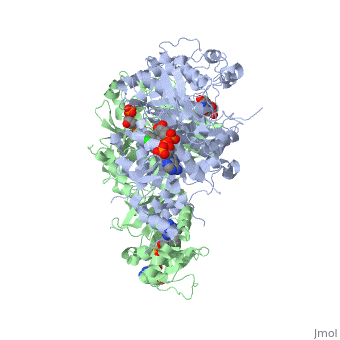
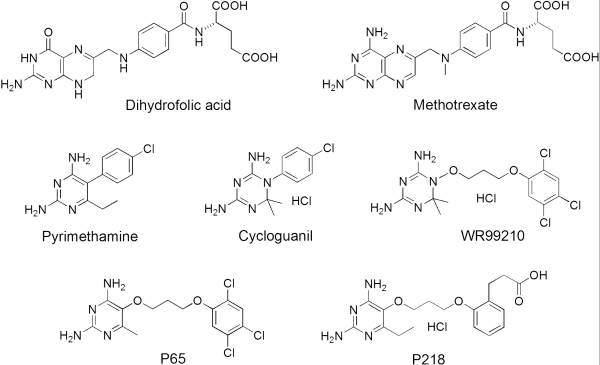
It was resolved that a 2,4-diaminopyrimidine anchor on a new drug would allow the steric hindrance found with pyrimethamine to be avoided by replacing its rigid chlorophenyl group. In addition to this anchor allowing deep binding into the active site of PfDHFR, the other end of the molecule, an alpha-carboxylate, would form strong hydrogen bonds with the conserved Arg122. | It was resolved that a 2,4-diaminopyrimidine anchor on a new drug would allow the steric hindrance found with pyrimethamine to be avoided by replacing its rigid chlorophenyl group. In addition to this anchor allowing deep binding into the active site of PfDHFR, the other end of the molecule, an alpha-carboxylate, would form strong hydrogen bonds with the conserved Arg122. | ||
| - | [[Image:Fig1.jpeg]] | + | [[Image:Fig1.jpeg]]<ref>Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</ref> |
Revision as of 07:46, 29 November 2012
Introduction
There are currently antimalarial drugs that target the malarial dihydrofolate reductase (DHFR) such as pyrimethamine and cycloguanil. However, the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. New research in drug development now incorporates both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain, the most lethal of the malaria species.[1]
| |||||||||||
References
- ↑ Somsak V, Uthaipibull C, Prommana P, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S. Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J. 2011 Oct 7;10:291. PMID: 21981896
- ↑ Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel