We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Josie N. Harmon/Sandbox Tutorial
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Mechanism of Action == | == Mechanism of Action == | ||
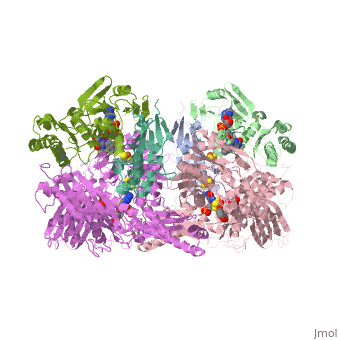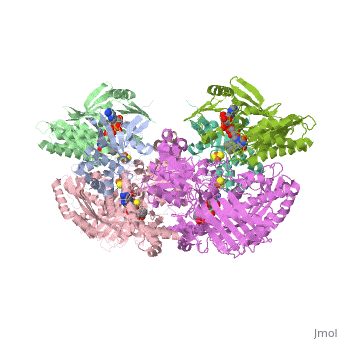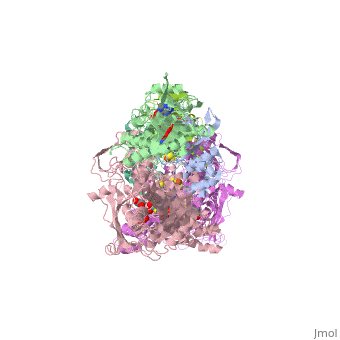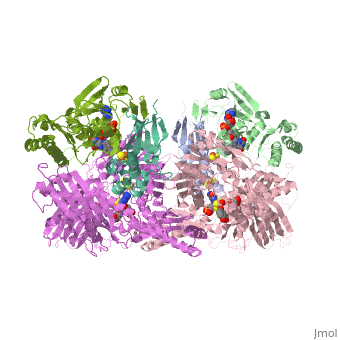
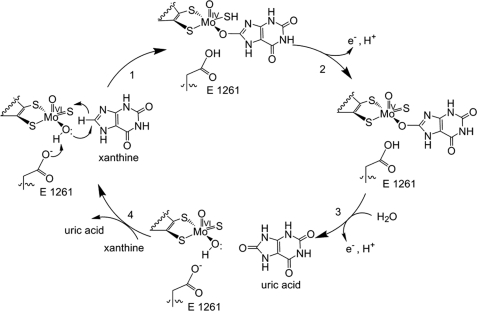
| - | Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. In eukaryotes xanthine oxidase exist as a <scene name='User:Josie_N._Harmon/Sandbox_1/Xanthine_oxidoreductase/3'>homodimer</scene> with each monomer containing <scene name='User:Josie_N._Harmon/Sandbox_1/Ligands_monomer/4'>four redox-active sites</scene>. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, <scene name='User:Josie_N._Harmon/Sandbox_1/Fe2s2_name/1'>Fe2S2</scene>, clusters, and <scene name='User:Josie_N._Harmon/Sandbox_1/Fad_name/1'>FAD</scene>. The <scene name='User:Josie_N._Harmon/Sandbox_1/Active_site/ | + | Xanthine oxidase is characterized as a molybdenum containing enzyme that catalyzes the hydroxylation of a sp2 hybrized carbon in a broad range of aromatic heterocycles and aldehydes. In eukaryotes xanthine oxidase exist as a <scene name='User:Josie_N._Harmon/Sandbox_1/Xanthine_oxidoreductase/3'>homodimer</scene> with each monomer containing <scene name='User:Josie_N._Harmon/Sandbox_1/Ligands_monomer/4'>four redox-active sites</scene>. The crystal structure of the bovine xanthine oxidase complex contains two active sites with varying intrinsic activity. The crystalline structure of a xanthine oxidase monomer offers a better view of the active molybdenum center, the ferredoxin iron sulfur, <scene name='User:Josie_N._Harmon/Sandbox_1/Fe2s2_name/1'>Fe2S2</scene>, clusters, and <scene name='User:Josie_N._Harmon/Sandbox_1/Fad_name/1'>FAD</scene>. The <scene name='User:Josie_N._Harmon/Sandbox_1/Active_site/3'>active site</scene> is thought to be composed of glutamine, glutamic acid, phenylalanine, arginine, and the molybdenum center. The substrate is believed to bind between the Phe 1009 and Phe 914. |
== Electron Extraction == | == Electron Extraction == | ||
Revision as of 16:05, 29 November 2012
Xanthine Oxidase Biochemistry Tutorial
The purpose of this tutorial is to explain the mechanism of the metabolic enzyme xanthine oxidoreductase.
| |||||||||||