Tutorial:Basic Chemistry Topics
From Proteopedia
| Line 51: | Line 51: | ||
| - | [[Image:Periodic table.png| thumb | center | 600px | | + | [[Image:Periodic table.png| thumb | center | 600px |<ref name="table">User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov.2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>. </ref>]] |
| Line 60: | Line 60: | ||
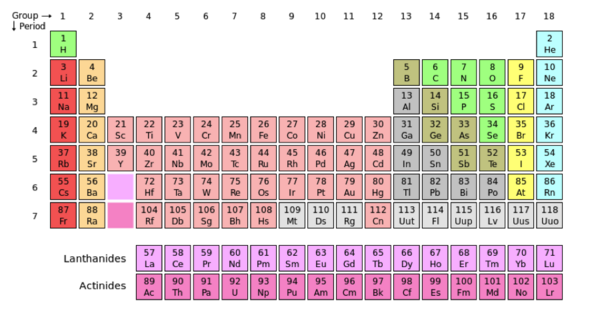
The image above displays Oxygen (O) in its periodic box. The atomic number of oxygen is 8, centered above the single-letter abbreviation of Oxygen. “8” represents the number of protons in the atom’s nucleus. The atomic number is also used to arrange elements in the periodic table. Notice the increasing value of protons in the atoms from left to right. The atomic mass of an element is centered under the single-letter abbreviation of an atom and represents the total mass of the atom. In this case, oxygen has an atomic mass of ~16. The total weight of an atom is the sum of the masses of its protons, neutrons and electrons. | The image above displays Oxygen (O) in its periodic box. The atomic number of oxygen is 8, centered above the single-letter abbreviation of Oxygen. “8” represents the number of protons in the atom’s nucleus. The atomic number is also used to arrange elements in the periodic table. Notice the increasing value of protons in the atoms from left to right. The atomic mass of an element is centered under the single-letter abbreviation of an atom and represents the total mass of the atom. In this case, oxygen has an atomic mass of ~16. The total weight of an atom is the sum of the masses of its protons, neutrons and electrons. | ||
| - | The periodic table is a collaboration of atoms that are arranged according to their common chemical features. Groups are the vertical columns of atoms, and the periods are the horizontal rows. When moving from left to right across the periods (horizontally), there is an increase in electronegativity. Electronegativity measures the attraction a bonded atom has for electrons. In other words, electronegativity refers a molecule's ability to pull other electrons towards it, increasing it’s density and providing itself with a negative charge. The concept of electronegativity contributes to the polarity of molecule. Polarity is caused by a difference in electronegativity between molecules in a compound or if the asymmetry of the compounds structure. A compound is nonpolar when the electronegativity of the molecules are close or even, the compound is symmetrical and the electrons are not being pulled more in one direction versus another. | + | The periodic table is a collaboration of atoms that are arranged according to their common chemical features. Groups are the vertical columns of atoms, and the periods are the horizontal rows. When moving from left to right across the periods (horizontally), there is an increase in electronegativity. Electronegativity measures the attraction a bonded atom has for electrons. In other words, electronegativity refers a molecule's ability to pull other electrons towards it, increasing it’s density and providing itself with a negative charge. The concept of electronegativity contributes to the polarity of molecule. Polarity is caused by a difference in electronegativity between molecules in a compound or if the asymmetry of the compounds structure. A compound is nonpolar when the electronegativity of the molecules are close or even, the compound is symmetrical and the electrons are not being pulled more in one direction versus another.<ref name="periodic table">"Periodic Table." Wikipedia. Wikipedia, n.d. Web. 16 Nov. 2012.<http://en.wikipedia.org/wiki/Periodic_table>.</ref> |
| - | + | ||
='''Amino acids'''= | ='''Amino acids'''= | ||
| - | Amino acids are the building blocks of proteins. There are about 500 amino acids, but the 20 more common amino acids will be referenced. Polymer chains (peptides) join amino acids together to form proteins. | + | Amino acids are the building blocks of proteins. There are about 500 amino acids, but the 20 more common amino acids will be referenced. Polymer chains (peptides) join amino acids together to form proteins. <ref name= "amino acids">"Amino Acids." Wikipedia. N.p., n.d. Web. 13 Oct. 2012. <http://en.wikipedia.org/wiki/Amino_acid>. </ref> |
'''20 More Common Animo Acids''' | '''20 More Common Animo Acids''' | ||
| Line 95: | Line 94: | ||
| - | The process of protein formation is known as translation. During translation, a complex known as the ribosome is responsible for adding amino acids to a peptide chain to form a complete protein. | + | The process of protein formation is known as translation. During translation, a complex known as the ribosome is responsible for adding amino acids to a peptide chain to form a complete protein. <ref name= "amino acids"/> |
[[Image:Amino Acid.png| thumb | left | 180px ]] | [[Image:Amino Acid.png| thumb | left | 180px ]] | ||
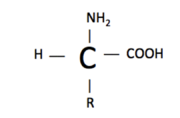
| - | An amino acid structure consists of an amine group (-NH2), a carboxylic acid (-COOH), hydrogen (H), and a functional group (R). The functional group is what varies between amino acids and determines how it is categorized. Amino acids are categorized as either essential/nonessential, polar/non-polar, or acidic/basic. | + | An amino acid structure consists of an amine group (-NH2), a carboxylic acid (-COOH), hydrogen (H), and a functional group (R). The functional group is what varies between amino acids and determines how it is categorized. Amino acids are categorized as either essential/nonessential, polar/non-polar, or acidic/basic. <ref name= "amino acids"/> |
| + | |||
| Line 113: | Line 113: | ||
'''Essential vs. Nonessentail Amino Aicds''' | '''Essential vs. Nonessentail Amino Aicds''' | ||
| - | An amino acid is considered essential when the human body is not capable of synthesizing/producing it. These amino acids need to be obtained from our diet. If the body is capable of producing an amino acid through metabolism or another method, the amino acid is classified as nonessential. | + | An amino acid is considered essential when the human body is not capable of synthesizing/producing it. These amino acids need to be obtained from our diet. If the body is capable of producing an amino acid through metabolism or another method, the amino acid is classified as nonessential.<ref name= "amino acids"/> |
| + | |||
<div style="float: left; width: 50%"> | <div style="float: left; width: 50%"> | ||
''Essential Amino Acids'' | ''Essential Amino Acids'' | ||
| Line 143: | Line 144: | ||
'''Polar vs. Nonpolar Amino Acids''' | '''Polar vs. Nonpolar Amino Acids''' | ||
| - | The polarity of an amino acid depends on the difference in electronegativity and the asymmetry of the compound's structure, discussed previously. For example, Arginine is a polar amino acid and Glycine is a nonpolar amino acid. Described earlier, an amino acid’s structure consists of a carboxylic acid, an amine, hydrogen, and a functional group. Look at the structure of <scene name='Tutorial:Basic_Chemistry_Topics/Polar_nonpolaraa/1'>arginine and glycine</scene>. Arginine has the amine group, carboxylic acid and hydrogen located towards the bottom of the representation, and the functional group is the large extension of atoms upward. You can see from this image that the functional group has a greater density/electronegativity compared to the core of the amino acid (carboxylic acid, amine and hydrogen), hence making this amino acid polar. In contrast the structure of glycine, located next to arginine, has little polarity. The functional group attached to glycine is only a methyl group (CH3). A methyl group has low density/electronegativity compared to the rest of the structure, making glycine a nonpolar amino acid. Neutral amino acids have functional groups that are similar in electronegativity compared to the core, so the electrons are not pulled in one direction more dominantly than another. In other words, there is no increase in density to one side. When an amino acid is neutral, it is less reactive than a polar amino acid. It is less reactive because the structure is stable. A polar amino acid is pulling electrons, yielding a slight positive and negative charge within the amino acid structure. This makes the compound less stable. The charges increase the molecules reactivity with other substances. | + | The polarity of an amino acid depends on the difference in electronegativity and the asymmetry of the compound's structure, discussed previously. For example, Arginine is a polar amino acid and Glycine is a nonpolar amino acid. Described earlier, an amino acid’s structure consists of a carboxylic acid, an amine, hydrogen, and a functional group. Look at the structure of <scene name='Tutorial:Basic_Chemistry_Topics/Polar_nonpolaraa/1'>arginine and glycine</scene>. Arginine has the amine group, carboxylic acid and hydrogen located towards the bottom of the representation, and the functional group is the large extension of atoms upward. You can see from this image that the functional group has a greater density/electronegativity compared to the core of the amino acid (carboxylic acid, amine and hydrogen), hence making this amino acid polar. In contrast the structure of glycine, located next to arginine, has little polarity. The functional group attached to glycine is only a methyl group (CH3). A methyl group has low density/electronegativity compared to the rest of the structure, making glycine a nonpolar amino acid. Neutral amino acids have functional groups that are similar in electronegativity compared to the core, so the electrons are not pulled in one direction more dominantly than another. In other words, there is no increase in density to one side. When an amino acid is neutral, it is less reactive than a polar amino acid. It is less reactive because the structure is stable. A polar amino acid is pulling electrons, yielding a slight positive and negative charge within the amino acid structure. This makes the compound less stable. The charges increase the molecules reactivity with other substances.<ref name= "amino acids"/> |
| + | |||
| Line 197: | Line 199: | ||
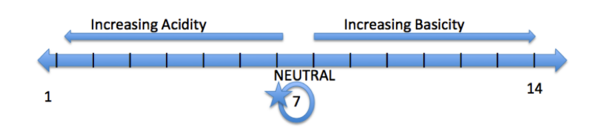
| - | An Example of a pH scale is located above. A pH scale ranges from 1-14, 1 being the most acidic and 14 being the most basic. An acidic pH has a value below 7, a basic pH is greater than 7, and a pH of 7 is considered neutral (ex: water). Some common acidic functional groups are alcohols (OH) and carboxylic acids (-COOH). Amines (-NH2) and ketones (-CH2OCH3) are considered basic functional groups. | + | An Example of a pH scale is located above. A pH scale ranges from 1-14, 1 being the most acidic and 14 being the most basic. An acidic pH has a value below 7, a basic pH is greater than 7, and a pH of 7 is considered neutral (ex: water). Some common acidic functional groups are alcohols (OH) and carboxylic acids (-COOH). Amines (-NH2) and ketones (-CH2OCH3) are considered basic functional groups.<ref name= "amino acids"/> |
| - | You can predict if an amino acid will be acidic or basic according to its structure. For example, <scene name='Tutorial:Basic_Chemistry_Topics/Glu/1'>glutamic acid</scene> contains a carboxylic acid functional group, which was stated previously to be an acidic functional group that lowers the pH of the amino acid. On the other hand, <scene name='Tutorial:Basic_Chemistry_Topics/Arg/1'>arginine</scene> is classified as a basic amino acid. The functional group of arginine contains a guanidine group. A guanidine group contains amine groups, which increase the pH of the amino acid. | + | You can predict if an amino acid will be acidic or basic according to its structure. For example, <scene name='Tutorial:Basic_Chemistry_Topics/Glu/1'>glutamic acid</scene> contains a carboxylic acid functional group, which was stated previously to be an acidic functional group that lowers the pH of the amino acid. On the other hand, <scene name='Tutorial:Basic_Chemistry_Topics/Arg/1'>arginine</scene> is classified as a basic amino acid. The functional group of arginine contains a guanidine group. A guanidine group contains amine groups, which increase the pH of the amino acid.<ref name= "amino acids"/> |
| + | |||
<div style="float: left; width: 50%"> | <div style="float: left; width: 50%"> | ||
| Line 224: | Line 227: | ||
The strongest of these bonds is the covalent bond. Covalent bonds involve sharing pairs of electrons between two atoms. These bonds are very stable. The figure shown below represents Coenzyme A (CoA) with the addition of an acyl group to the sulfur atom. The introduction mentioned that the addition of the acetyl group is important to the study, because when it is transferred to Tobramycin the antibiotic becomes inactive. The solid connections between the atoms are representation of the covalent bonds. | The strongest of these bonds is the covalent bond. Covalent bonds involve sharing pairs of electrons between two atoms. These bonds are very stable. The figure shown below represents Coenzyme A (CoA) with the addition of an acyl group to the sulfur atom. The introduction mentioned that the addition of the acetyl group is important to the study, because when it is transferred to Tobramycin the antibiotic becomes inactive. The solid connections between the atoms are representation of the covalent bonds. | ||
| - | [[Image:CoA + Acyl group.png | thumb | center | 300px | Covalent bonding]] | + | [[Image:CoA + Acyl group.png | thumb | center | 300px | Covalent bonding]] |
==Ionic Bonds== | ==Ionic Bonds== | ||
| Line 230: | Line 233: | ||
[[Image:Ionic bond.png| thumb | center | 400px | Ionic Bonding<ref>. "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.</ref>]] | [[Image:Ionic bond.png| thumb | center | 400px | Ionic Bonding<ref>. "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.</ref>]] | ||
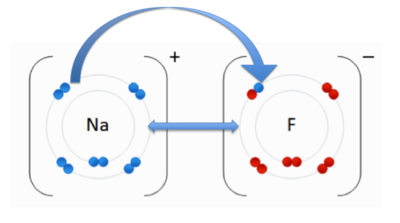
| - | An ionic bond occurs when an electron(s) is transferred from one atom to another to become more stable with a full electron shell. The charges result from the transfer of an electron. The positively charged cation is attracted to the negatively charged anion. In the image to the right, you see an anion Fluoride (F-), and the cation Sodium (Na+). The double-sided arrow between them is representation of their attractive force. Fluorine has a higher electronegativity than sodium. As we discussed previously, when an atom has higher electronegativity it pulls electrons from the lower electronegative atom; in this case sodium. The transfer of the sodium electron (blue) is shown using an arrow. | + | An ionic bond occurs when an electron(s) is transferred from one atom to another to become more stable with a full electron shell. The charges result from the transfer of an electron. The positively charged cation is attracted to the negatively charged anion. In the image to the right, you see an anion Fluoride (F-), and the cation Sodium (Na+). The double-sided arrow between them is representation of their attractive force. Fluorine has a higher electronegativity than sodium. As we discussed previously, when an atom has higher electronegativity it pulls electrons from the lower electronegative atom; in this case sodium. The transfer of the sodium electron (blue) is shown using an arrow. <ref name="ionic bonds">User:Cepheus. "Ionic Bonds." Wikipedia. N.p., n.d. Web. 13 Nov. 2012. <http://en.wikipedia.org/wiki/Ionic_bond>. </ref> |
Tobramycin and aspartic acid form an <scene name='Tutorial:Basic_Chemistry_Topics/New_ionic_bond/1'>ionic bond</scene> in this representation. The nitrogen on tobramycin has a (+) positive charge and aspartic acid has a (-) negative charge. The opposing charges are attracted to each other forming an ionic bond, holding the compounds in close proximity. | Tobramycin and aspartic acid form an <scene name='Tutorial:Basic_Chemistry_Topics/New_ionic_bond/1'>ionic bond</scene> in this representation. The nitrogen on tobramycin has a (+) positive charge and aspartic acid has a (-) negative charge. The opposing charges are attracted to each other forming an ionic bond, holding the compounds in close proximity. | ||
Revision as of 03:43, 3 December 2012
Purpose of the Tutorial
- This tutorial is intended as a beneficial learning/teaching aid for an entry-level chemistry college student with some basic chemistry knowledge. Various general chemistry concepts are explained using a research article as an example. Applying general chemistry to a research article will allow the students to see the impact they can have on the research world in the future by applying their knowledge.
Summary: Scientific Research Article
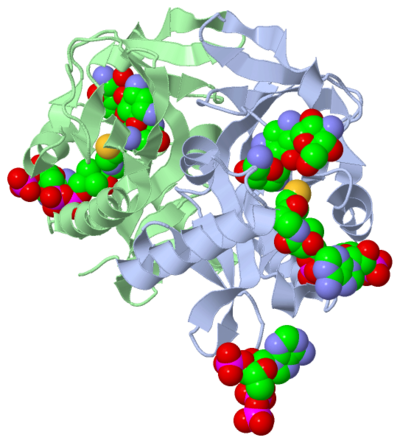
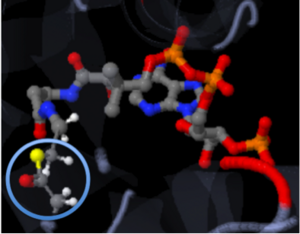
The molecule to left is from the article "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin" published in Nature Structural Biology.[2]. The study focused on aminoglycoside 2’- N- acetyltransferase (AAC (2’)- Ic), an enzyme. This enzyme is a protein that speeds the rate of the reaction it catalyzes.
This study determined the structure of AAC (2’)-Ic from mycobacterium tuberculosis, a pathogen. This pathogen is a microorganism that causes tuberculosis (TB), which typically affects the lungs, but can affect other parts of the body as well. The specific structure/protein fold of AAC (2’)-Ic places it in the GCN5-related N-acetyltransferase (GNAT) superfamily. The GNAT superfamily is a group of enzymes that are similar in structure. The protein fold is important because it determines the function of the compound.[2]
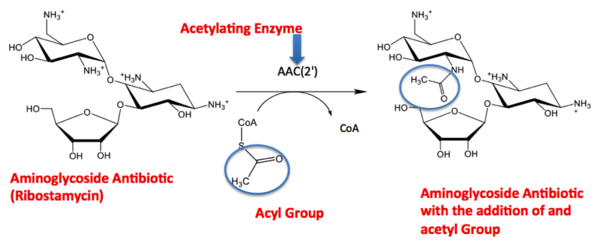
The GNAT family is a group of acetylating enzymes. Acetylation is the addition of CH3CO functional group onto a compound. Although the physiological function of AAC(2’)-Ic is not certain, the discovery of the GNAT fold allowed researchers to classify AAC (2’)-Ic as an acetylating enzyme. Mycothiol is catalyzed by AAC (2’)-Ic to acetylate the aminoglycoside antibiotic known as tobramycin. When this occurs the aminoglycoside antibiotic becomes inactive. The basis of this study is important because when pathogens become resistant to commonly used antibiotics, an infection that was easily cured can now become severe and life threatening.[2]
| |||||||||||
References
- ↑ Vetting, M. W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin." RCSB Protien DataBase. N.p., 28 Aug.2002. Web. 13 July 2011. http://www.rcsb.org/pdb/explore/explore.do?structureId=1M4D
- ↑ 2.0 2.1 2.2 2.3 2.4 Vetting, Matthew W., et al. "Aminoglycoside 2'-N-acetyltransferase from Mycobacterium tuberculosis-Complex with Coenzyme A and Tobramycin."Nature Structural Biology 9.9 (2002): 653-58. Print.
- ↑ User:Cepheus. "Periodic Table." Wikipedia. N.p., 26 Feb. 2007. Web. 26 Nov.2012. <http://en.wikipedia.org/wiki/File:Periodic_table.svg>.
- ↑ "Periodic Table." Wikipedia. Wikipedia, n.d. Web. 16 Nov. 2012.<http://en.wikipedia.org/wiki/Periodic_table>.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Amino Acids." Wikipedia. N.p., n.d. Web. 13 Oct. 2012. <http://en.wikipedia.org/wiki/Amino_acid>.
- ↑ . "File:NaF.gif." Wikipedia. Wikipedia, 17 June 2011. Web. 31 Oct. 2012.<http://en.wikipedia.org/wiki/File:NaF.gif.
- ↑ User:Cepheus. "Ionic Bonds." Wikipedia. N.p., n.d. Web. 13 Nov. 2012. <http://en.wikipedia.org/wiki/Ionic_bond>.
- ↑ Maňas, Michal, trans. "File:3D model hydrogen bonds in water.jpg." Wikimedia Commons. Wikimedia Commons, 3 Dec. 2007. Web. 31 Oct. 2012 <http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg.
- ↑ Wikipedia. Wikipedia, 4 Nov. 2012. Web. 7 Nov. 2012. <http://en.wikipedia.org/wiki/Enzyme_substrate_(biology)
- ↑ "Tobramycin." Wikipedia. Wikipedia, n.d. Web. 26 Nov. 2012.<http://en.wikipedia.org/wiki/Tobramycin>.