We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hepatocyte growth factor receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=== Hepatocyte Growth Factor Receptor === | === Hepatocyte Growth Factor Receptor === | ||
| + | |||
| + | <StructureSection load='1r1w' size='400' frame='true' align='right' scene='1r1w/Common_view/3' > | ||
==Introduction== | ==Introduction== | ||
| Line 6: | Line 8: | ||
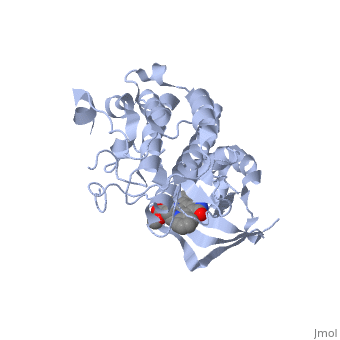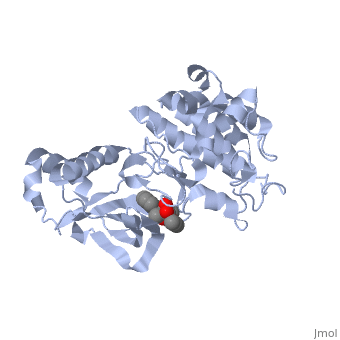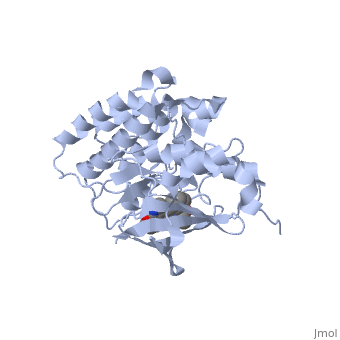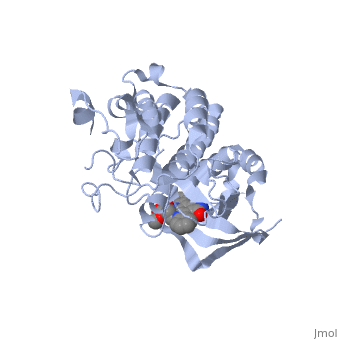
==Structure== | ==Structure== | ||
| - | {{STRUCTURE_1r1w| PDB=1r1w | SCENE= }} | ||
Some of the most important aspects of this RTK are the tyrosines at position 1234 and 1235. These two residues will become phosphorylated upon activation of the protein. There is another pair of important <scene name='User:Juliette_Personius/sandbox_1/Tyrosines_1349_and_1356/1'>tyrosine residues (1349 and 1356)</scene> . Studies with mice have shown that these tyrosines are necessary for normal c-met signaling. When these two tyrosines were substituted with with phenylalenine, the mice had an embryonically lethal phenotype and defects were found in placenta, liver, muscles and nerves. <ref>PMID: 8898205</ref> In a wild type c-met, these sites will become phosphorylated and act as docking sites for many different transducers and adapters. <ref>PMID: 14559966</ref> The c-met kinase domain is very similar to a typical protein kinase structure. | Some of the most important aspects of this RTK are the tyrosines at position 1234 and 1235. These two residues will become phosphorylated upon activation of the protein. There is another pair of important <scene name='User:Juliette_Personius/sandbox_1/Tyrosines_1349_and_1356/1'>tyrosine residues (1349 and 1356)</scene> . Studies with mice have shown that these tyrosines are necessary for normal c-met signaling. When these two tyrosines were substituted with with phenylalenine, the mice had an embryonically lethal phenotype and defects were found in placenta, liver, muscles and nerves. <ref>PMID: 8898205</ref> In a wild type c-met, these sites will become phosphorylated and act as docking sites for many different transducers and adapters. <ref>PMID: 14559966</ref> The c-met kinase domain is very similar to a typical protein kinase structure. | ||
The N-terminal of the protein contains many <scene name='User:Juliette_Personius/sandbox_1/Beta_sheets/2'>β-sheets </scene> and is linked through a hinge to the C lobe, which is full of α helices. This particular kinase domain is very similar to the domains of the insulin receptor kinase and fibroblast growth factor receptor kinase. One main difference is that the c-met structure has an helix between residues 1060-1069 not present in FGFRK or IRK. <ref>PMID: 14559966</ref> | The N-terminal of the protein contains many <scene name='User:Juliette_Personius/sandbox_1/Beta_sheets/2'>β-sheets </scene> and is linked through a hinge to the C lobe, which is full of α helices. This particular kinase domain is very similar to the domains of the insulin receptor kinase and fibroblast growth factor receptor kinase. One main difference is that the c-met structure has an helix between residues 1060-1069 not present in FGFRK or IRK. <ref>PMID: 14559966</ref> | ||
| Line 16: | Line 17: | ||
==Mutation== | ==Mutation== | ||
| - | {{STRUCTURE_1r0p| PDB=1r0p | SCENE= }} | ||
| - | |||
This particular structure of the hepatocyte growth factor tyrosine kinase domain is one harboring a human cancer mutation. The two | This particular structure of the hepatocyte growth factor tyrosine kinase domain is one harboring a human cancer mutation. The two | ||
<scene name='User:Juliette_Personius/sandbox_1/1234_and_1235_mutations/1'>tyr1234 and tyr1235</scene> are replaced by a phenylalanine and aspartate, respectively. This mutation normally causes the receptor to be consitutively active, and is found in metastatic HNSC carcinoma. Although there is no longer phosphorylation at these sites, it is believed that the aspartate negative charge resembles the negative phosphate that would normally cause activation, and therefore keeps the protein in its active form. <ref>PMID: 14559966</ref> There is a third mutation at Tyr-1194 which is substituted for a phenylalanine. This is shown to point in a the pocket formed by Lys-1198 and Leu-1195 from αE. <ref>PMID: 14559966</ref> This structure is conserved in the wild | <scene name='User:Juliette_Personius/sandbox_1/1234_and_1235_mutations/1'>tyr1234 and tyr1235</scene> are replaced by a phenylalanine and aspartate, respectively. This mutation normally causes the receptor to be consitutively active, and is found in metastatic HNSC carcinoma. Although there is no longer phosphorylation at these sites, it is believed that the aspartate negative charge resembles the negative phosphate that would normally cause activation, and therefore keeps the protein in its active form. <ref>PMID: 14559966</ref> There is a third mutation at Tyr-1194 which is substituted for a phenylalanine. This is shown to point in a the pocket formed by Lys-1198 and Leu-1195 from αE. <ref>PMID: 14559966</ref> This structure is conserved in the wild | ||
Revision as of 02:11, 4 December 2012
Hepatocyte Growth Factor Receptor
| |||||||||||