Circadian Clock Protein KaiC
From Proteopedia
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[Image:KaiCABinteraction.jpg | thumb | alt=text | Interactions of KaiC with KaiA and KaiB ]] | [[Image:KaiCABinteraction.jpg | thumb | alt=text | Interactions of KaiC with KaiA and KaiB ]] | ||
| - | Biological Circadian Clocks are self-sustaining oscillators that function on a rhythmic cycle of or around 24 hours. The are found in almost all organisms, the simplest of which are cyanobacteria, which have been extensively studied in order to determine the mechanism of the fine-tuned biological process of circadian rhythmicity. In most eukaryotes, a region of the brain called the superchiasmic nuclei detects light and dark cycles that relay the message to biological clock systems that maintain rhythmicity within the body. Conversely, cyanobacteria have a fairly small system comprised of three proteins: KaiC, KaiA, and KaiB. The system is based around the central protein KaiC which exhibits ATP binding, inter-subunit organization, a scaffold region for Kai protein complex formation, a location where critical mutations are found, and an evolutionary link to other well-known proteins | + | Biological Circadian Clocks are self-sustaining oscillators that function on a rhythmic cycle of or around 24 hours. The are found in almost all organisms, the simplest of which are cyanobacteria, which have been extensively studied in order to determine the mechanism of the fine-tuned biological process of circadian rhythmicity. In most eukaryotes, a region of the brain called the superchiasmic nuclei detects light and dark cycles that relay the message to biological clock systems that maintain rhythmicity within the body. Conversely, cyanobacteria have a fairly small system comprised of three proteins: KaiC, KaiA, and KaiB. The system is based around the central protein KaiC which exhibits ATP binding, inter-subunit organization, a scaffold region for Kai protein complex formation, a location where critical mutations are found, and an evolutionary link to other well-known proteins <ref>PMID: 15304218</ref>. |
==KaiC - KaiA - KaiB System== | ==KaiC - KaiA - KaiB System== | ||
| Line 8: | Line 8: | ||
== KaiC Homohexameric Complex == | == KaiC Homohexameric Complex == | ||
<StructureSection load='1TF7' size='500' side='left' caption='Structure of KaiC (PDB entry [[1TF7]])' scene=''> | <StructureSection load='1TF7' size='500' side='left' caption='Structure of KaiC (PDB entry [[1TF7]])' scene=''> | ||
| - | The structure of KaiC resembles a double donut formation with a central pore through the center. Conceptually, it is comprised of two subunits, <scene name='Circadian_Clock_Protein_KaiC/Ci_in_blue_and_cii_in_pink/1'>CI (blue) and CII (pink)</scene> which appear as the two donuts. Yet, technically, the protein divided perpendicular to the conceptual lateral subdivision, which can be visualized as six <scene name='Circadian_Clock_Protein_KaiC/Chain_a_of_hexamer/1'>homologous monomers</scene> that form a barrel shaped structure. The entire molecule is made up of 519 amino acid residues in each monomer (, and spans 100 Angstroms in diameter. The waist of the molecule (the region between CI and CII) has a diameter of 62 Angstroms. The <scene name='Circadian_Clock_Protein_KaiC/Central_pore/2'>central pore</scene> that runs through the molecule is wider at the CI region, 22 Angstroms, and narrows to a nearly <scene name='Circadian_Clock_Protein_KaiC/Surface_view_-_central_pore/1'>closed conformation</scene> that appears to be secured by six <scene name='Circadian_Clock_Protein_KaiC/Surface_view_central_pore-arg/2'>arginine</scene> residues. The basic, polar nature and physical divergence of this CII region in comparison to the CI end indicates the possibility of conformational change, such as closing and opening. | + | The structure of KaiC resembles a double donut formation with a central pore through the center. Conceptually, it is comprised of two subunits, <scene name='Circadian_Clock_Protein_KaiC/Ci_in_blue_and_cii_in_pink/1'>CI (blue) and CII (pink)</scene> which appear as the two donuts. Yet, technically, the protein divided perpendicular to the conceptual lateral subdivision, which can be visualized as six <scene name='Circadian_Clock_Protein_KaiC/Chain_a_of_hexamer/1'>homologous monomers</scene> that form a barrel shaped structure. The entire molecule is made up of 519 amino acid residues in each monomer (, and spans 100 Angstroms in diameter. The waist of the molecule (the region between CI and CII) has a diameter of 62 Angstroms. The <scene name='Circadian_Clock_Protein_KaiC/Central_pore/2'>central pore</scene> that runs through the molecule is wider at the CI region, 22 Angstroms, and narrows to a nearly <scene name='Circadian_Clock_Protein_KaiC/Surface_view_-_central_pore/1'>closed conformation</scene> that appears to be secured by six <scene name='Circadian_Clock_Protein_KaiC/Surface_view_central_pore-arg/2'>arginine</scene> residues. The basic, polar nature and physical divergence of this CII region in comparison to the CI end indicates the possibility of conformational change, such as closing and opening <ref>PMID: 15304218</ref>. |
There are 12 molecules of <scene name='Circadian_Clock_Protein_KaiC/Highlight_atp/1'>ATP</scene> bound at the interface between monomers. Three potential phosphorylation sites have been identified within 10 Angstroms of the <scene name='Circadian_Clock_Protein_KaiC/Active_site/1'>ATP binding region</scene>. | There are 12 molecules of <scene name='Circadian_Clock_Protein_KaiC/Highlight_atp/1'>ATP</scene> bound at the interface between monomers. Three potential phosphorylation sites have been identified within 10 Angstroms of the <scene name='Circadian_Clock_Protein_KaiC/Active_site/1'>ATP binding region</scene>. | ||
Revision as of 06:31, 4 December 2012
Contents |
Introduction
Biological Circadian Clocks are self-sustaining oscillators that function on a rhythmic cycle of or around 24 hours. The are found in almost all organisms, the simplest of which are cyanobacteria, which have been extensively studied in order to determine the mechanism of the fine-tuned biological process of circadian rhythmicity. In most eukaryotes, a region of the brain called the superchiasmic nuclei detects light and dark cycles that relay the message to biological clock systems that maintain rhythmicity within the body. Conversely, cyanobacteria have a fairly small system comprised of three proteins: KaiC, KaiA, and KaiB. The system is based around the central protein KaiC which exhibits ATP binding, inter-subunit organization, a scaffold region for Kai protein complex formation, a location where critical mutations are found, and an evolutionary link to other well-known proteins [1].
KaiC - KaiA - KaiB System
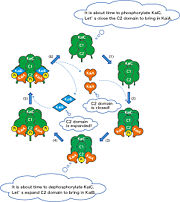
KaiC is the central clock protein, which has autokinase and autophosphorylase activity. Yet in the presence of ATP, KaiC cannot perform it's autophosphorylation function. It requires two other proteins, KaiA and KaiB, the genes of which are found in the same cluster on the chromosome [2]. Although KaiC phosphorylates itself, the presence of KaiA and KaiB are essential to rhythmicity. KaiA stimulates KaiC autophosphorylation, while KaiB antagonizes the process possibly by enhancing KaiC dephosphorylation. Even in the presence of high ATP, KaiB still prompts KaiC to dephosphorylate.
KaiC Homohexameric Complex
| |||||||||||
KaiA <-> KaiC Interaction Site
(show 3D image of KaiC site for KaiA binding and highlight key residues in interaction -weak? strong? any ions in site? how does it stabilize autophosporylation?) KaiA binds to the interface of the two donut-shaped KaiC subunits, CI and CII. This area, known as the "waist" of the molecule
KaiB <-> KaiC Interaction Site
(show 3D image of KaiC site for KaiB binding and highlight key residues in interaction -weak? strong? ions in site? how does it stabilize dephosphorylation/destabilize phosphorylation/destabilize KaiA?)
Biological Importance
- Nearly all promoters in a cyanobacteria are under circadian control. [function is important to whole life cycle] (1)
References
- ↑ Pattanayek R, Wang J, Mori T, Xu Y, Johnson CH, Egli M. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol Cell. 2004 Aug 13;15(3):375-88. PMID:15304218 doi:10.1016/j.molcel.2004.07.013
- ↑ Pattanayek R, Wang J, Mori T, Xu Y, Johnson CH, Egli M. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol Cell. 2004 Aug 13;15(3):375-88. PMID:15304218 doi:10.1016/j.molcel.2004.07.013
- ↑ Pattanayek R, Wang J, Mori T, Xu Y, Johnson CH, Egli M. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol Cell. 2004 Aug 13;15(3):375-88. PMID:15304218 doi:10.1016/j.molcel.2004.07.013
Proteopedia Page Contributors and Editors (what is this?)
Ashley Beechan, Michal Harel, Alexander Berchansky, Jaime Prilusky