SandboxPKA
From Proteopedia
| Line 11: | Line 11: | ||
The fusion of the gene encoding c-Abl with the breakpoint cluster region (BCR) gene, results in the formation of a fusion protein, BCR-Abl, in which all of c-Abl is preserved without mutation, except for the “cap” region upstream of the SH3 domain ('''figure 1b'''). | The fusion of the gene encoding c-Abl with the breakpoint cluster region (BCR) gene, results in the formation of a fusion protein, BCR-Abl, in which all of c-Abl is preserved without mutation, except for the “cap” region upstream of the SH3 domain ('''figure 1b'''). | ||
| - | [[Image:figura1jc.jpg]] | + | [[Image:figura1jc.jpg|thumb|left]] |
Revision as of 22:16, 9 December 2012
The c-Abl protein 1 (ABL1), also known as Abelson kinase, is a non-receptor tyrosine kinase that plays a role in many key processes linked to cell growth and survival such as cytoskeleton remodeling in response to extracellular stimuli, cell motility and adhesion, receptor endocytosis, autophagy, DNA damage response and apoptosis. [1] [2] Activity of c-Abl protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns ABL1 into an oncogene. In more than 90% cases, chronic myelogeneous leukemia (CML) is caused by a chromosomal abnormality that results in the formation of the Philadelphia chromosome. This chromosome is formed by fusion between Abelson (Abl) tyrosine kinase gene at chromosome 9 and break point cluster (BCR) gene at chromosome 22, resulting in the chimeric oncogene BCR-Abl and a constitutively active BCR-Abl tyrosine kinase. Small molecule inhibitors of BCR-Abl that bind to the kinase domain can be used to treat CML [3]
Contents |
From the Sequence to the Structure
Eukayotic protein kinases evolved as a family of highly dynamic molecules with strictly organized internal architecture. C-Abl consists of 1150 residues. The N-terminal half of the protein includes an N-terminal “cap” of 80 residues that is important for autoinhibition, followed by an SH3 domain, an SH2 domain, and a tyrosine kinase domain. The C-terminal half of c-Abl includes binding elements for SH3 domains, nuclear localization and export signals, a DNA binding functionality, and an actin binding domain. Human cells express two splice variants of c-Abl, Abl 1a and Abl 1b, which differ only in the very N-terminal region. Abl 1b is myristoylated, whereas Abl1a is not (figure 1a).
The fusion of the gene encoding c-Abl with the breakpoint cluster region (BCR) gene, results in the formation of a fusion protein, BCR-Abl, in which all of c-Abl is preserved without mutation, except for the “cap” region upstream of the SH3 domain (figure 1b).
All of the protein kinases have a similar bilobal fold, and their key structural features have been well studied. Like others, the abelson kinase incorporates a highly conserved bi-lobed structure with an adenosine triphosphate (ATP) binding domain situated in a deep cleft between the N- and C-terminal lobes. Adjacent to this is the centrally located activation loop that incorporates a conserved Asp-Phe-Gly (DFG) sequence and controls catalytic activity by switching between different states in a phosphorylation-dependent manner [4].
| |||||||||||
Bcr-Abl tyrosine kinase
|
The human Bcr protein contains 1,271 amino acids and multiple domains, including the oligomerization domain (OLI), the serine/threonine kinase domain (S/TK), the domain homologous to the human Dbl and yeast Cdc24 proteins (DH) and the domain with GTPase-activating activity for Rac (RacGAP). The c-Abl protein contains 1,097 amino acids, which include the Src-homology domains 3/2 (SH3/SH2), tyrosine kinase domain (TK), nuclear translocalization signal (NTS), DNA binding domain (DB) and actin-binding motif (AB). Two domains are essential for transforming activity — OLI from Bcr and TK from Abl. Depending on the site of the breakpoint in the Bcr gene, the fusion protein can vary in size from 190 to 230 kDa. Each fusion protein contains the same portion of the c-Abl protein but differs in the length of the Bcr portion. [5]
Normal ABL has a tri-dimensional structure which is tightly preserved in a closed, inactive conformation order to prevent oncogenic activation. The maintenance of this inactive conformation is possible by:
1- The "latching" of the myristilated NH2-terminal sequence which is directly linked to a myristilation recognition sequence on the c-lobe of the SH1 kinase domain,
2- The close contact between SH3 and SH2 domain,
3- The interactions between SH3 domain and the C-lobe of the kinase domain. These interactions clamp the structure and prevent the kinase to switch to an active conformation, a process which requires the phosphorylation of Tyr 412 residue and the "unlatching" of the myristoyl group from the C-Lobe of the kinase domain. The attachment of proline-rich SH2 and SH3 ligands leads to the complete switch of the protein to an open, active conformation of the kinase. The NH2-terminal myristilation (autoregulatory role) is deleted during the t(9;22) translocation. [6]
Bcr-Abl tyrosine-kinase inhibitors
Gleevec
Imatinib mesylate (STI571 or Gleevec), discovered in 1992, is a Bcr-Abl tyrosine kinase inhibitor (Bcr-Abl TKI) and it's the most common first generation drug used for the CML treatment. Imatinib has a high affinity for ABL kinase.
Imatinib achieves BCR-ABL kinase inhibition by binding to the inactive, unphosphorylated conformation of the kinase (DFG-out), thereby reducing the availability of the catalytically active phosphorylated conformation (DFG-in), necessary for ATP binding. This blocks signal transduction, ultimately resulting in inhibition of proliferation and loss of viability and proliferation.[7].
Imatinib is used for treating CML, gastrointestinal stromal tumours and other diseases. By 2011, Gleevec has been FDA approved to treat ten different cancers.
Pharmacokinetics: Imatinib is rapidly absorbed when given by mouth, and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system. The main metabolite, N-demethylated piperazine derivative, is also active. The half-lives of imatinib and its main metabolite are 18 and 40 hours, respectively.
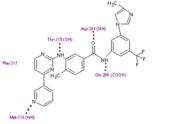
Nilotinib
Nilotinib (AMN107) is a second-generation oral TKI. It was designed based on the crystal structure of imatinib to be highly active against a wide range of imatinib-resistant BCR-ABL mutants and is approved for the treatment of newly diagnosed or imatinib-resistant or -intolerant CML. nilotinib was designed to maintain binding to the inactive conformation of the ABL kinase domain, while incorporating alternative binding groups to the N-methylpiperazine moiety and preserving an amide pharmacophore to retain H-bond interactions with Glu286 and Asp381. [8] Substitution of the N-methylpiperazine moiety present in imatinib by a phenyl group bearing trifluoromethyl and imidazole substituents in the nilotinib structure greatly contributes to the potency of nilotinib by reducing the requirement for hydrogen bonding with nilotinib (four H-bond interactions compared with six H-bonds for imatinib). Among patients with imatinib resistant CML, nilotinib has not been associated with the toxic effects commonly seen with imatinib treatment, such as fluid retention, edema, and weight gain [9]
Dasatinib
Dasatinib is also a second-generation TKI. Dasatinib binds kinases of the SCR family (0,2 nM<IC50<1,1nM). It binds both the active and inactiv forms of the Abl kinase with a higher affinity (300 times) than Imatinib. Dasatinib is active against most BCR-ABL mutants with the exception of T315I. [10] Dasatinib is well tollerated but adverse effects can include muscle and joint aches, fatigue, dermatologic complaints such as rash and acne, headaches, and diarrhea [11]
Third generation drugs
New mutations of Bcr-Abl tyrosine kinase are still being discovered and some old go unconquered. To overcome those mutations, new generation drugs are being developed to treat patients with CML.
Bosutinib (SKI-606)
On September 4, 2012, the U. S. Food and Drug Administration approved bosutinib tablets (Bosulif, Pfizer, Inc.) for the treatment of chronic, accelerated, or blast phase Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) in adult patients with resistance or intolerance to prior therapy. [12]
is based on a quinoline scaffold and is structurally related to the AstraZeneca quinazoline template. [13]
Ponatinib (AP24534)
Ponatinib was identified using structure base drug design and focused synthetic libraries of trisubstituted purine analogs. The substance potently inhibits, on nanomolar scale, Src and Bcr-Abl kinases including many common imatinib resistant Bcr-Abl mutations, like T315I mutation. The key structural feature of the molecule is a carbon-carbon triple bond linkage that makes productive hydrophobic contact with the side chain of I315, allowing inhibition of the T315I mutant. The triple bond also acts as an inflexible connector that enforces correct positioning of the two binding segments of AP24534 into their established binding pockets. AP24534 maintains an extensive hydrogen-bonding network and occupies a region of the kinase that overlaps significantly with the imatinib binding site. [14]
Reaction
Protein kinases are a group of enzymes that possess a catalytic subunit that transfers the gamma (terminal) phosphate from nucleotide triphosphates (often ATP) to one or more amino acid residues in a protein substrate side chain, resulting in a conformational change affecting side protein function.
The enzymes are classified into two broad groups, characterised with respect to substrate specificity:
- Serine/threonine kinases
- Tyrosine specific kinases: c-Abl is included in this group [15]
Catalytic domain
It is responsible of both, ATP binding as well as protein binding.
| |||||||||||
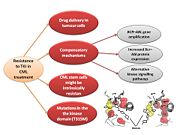
Resistance
In the majority of cases, resistance is caused by reactivation of Bcr–Abl kinase activity. The F-helix and two hydrophobic spines define the internal architecture of the protein kinase molecule. The R-spine (red surface) and C-spine (yellow surface)are anchored to the F-helix in the middle of the rigid C-lobe. Major elements of the catalytic machinery are also anchored to the F-helix directly or via the spines. In Abl kinase, the gatekeeper is a smaller threonine (Thr 315) that is not an effective stabilizer of the R-spine. Mutation in this residue is the most common mechanism implicated in secondary drug resistance. Usually, gatekeeper threonine is substituted by isoleucine or methionine and avoid Gleevec entrance to ATP-binding domain [16].
In some treated patients, BCR–ABL gene amplification and increased Bcr–Abl protein expression have been observed as a compensatory mechanism for the imatinib antitumour effect. The switch to alternative kinase signalling pathways, which can compensate for the loss of Bcr–Abl activity, has been proposed as another resistance strategy of leukaemic cells. Imatinib might also have different effects on chronic myelogenous leukaemia (CML) tumour cells depending on their differentiation state, and it has been proposed that quiescent CML stem cells might be intrinsically resistant to the drug. Other mechanisms of resistance to imatinib might be related to pharmacokinetic factors of drug delivery. Imatinib can be actively transported out of tumour cells through efflux pump proteins to keep intracellular drug concentrations below inhibitory levels. Extracellular sequestration of imatinib by 1 acid glycoprotein in the plasma has been proposed as a potential mechanism, which would result in reduced availability of the free drug to CML cells [17] .
| |||||||||||
References
- ↑ Yuan ZM, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1437-40. PMID:9037071
- ↑ Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000 Nov 20;19(49):5643-50. PMID:11114745 doi:10.1038/sj.onc.1203878
- ↑ Crystal Structures of the Kinase Domain of c-Abl in Complex with the Small Molecule Inhibitors PD173955 and STI571
- ↑ Schindler T, Bornmann W, Pellicena P, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938 – 42.
- ↑ Zhao X, Ghaffari S, Lodish H, Malashkevich VN, Kim PS. Structure of the Bcr-Abl oncoprotein oligomerization domain. Nat Struct Biol. 2002 Feb;9(2):117-20. PMID:11780146 doi:10.1038/nsb747
- ↑ http://atlasgeneticsoncology.org/Genes/ABL.html
- ↑ Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006;2:358 – 64.
- ↑ Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129 – 41.
- ↑ Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosomepositive ALL. N Engl J Med. 2006;354:2542–51.
- ↑ Shah NP, Tran C, Lee FY, et al. (2004) Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305:399–401.
- ↑ Results of Dasatinib Therapy in Patients With Early Chronic-Phase Chronic Myeloid Leukemia Jorge E. Cortes, Dan Jones, Susan O'Brien, Elias Jabbour, Farhad Ravandi, Charles Koller, Gautam Borthakur, Brenda Walker, Weiqiang Zhao, Jianqin Shan and Hagop Kantarjian
- ↑ http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm318203.htm
- ↑ Manley, P.; Cowan-Jacob, S.; Mestan, J. (2005). "Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia". Biochimica et Biophysica Acta 1754 (1–2): 3–13.
- ↑ O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009 Nov 6;16(5):401-12. PMID:19878872 doi:10.1016/j.ccr.2009.09.028
- ↑ Leukemia research 34 (10): 1255–1268. doi:10.1016/j.leukres.2010.04.016. PMID 2053738
- ↑ Protein kinases: evolution of dynamic regulatory proteins
- ↑ http://www.nature.com/nrd/journal/v3/n12/full/nrd1579.html