Sandbox Reserved 715
From Proteopedia
| Line 17: | Line 17: | ||
===The Glycoside Hydrolysases=== | ===The Glycoside Hydrolysases=== | ||
| - | + | Glycoside hydrolases (GH) constitute a widespread enzyme group presenting a huge variety of protein folds and substrate specificities. They are classified into EC 3.2.1 as enzymes catalyzing the hydrolysis of O- or S-glycosides. The enzymatic hydrolysis of glycosidic bonds can be realised by two ways : inversion or retention of the product anomeric configuration compared with the substrate anomeric configuration. So we can classify Glycoside hydrolases according to these mechanismes. But we can alson make a classification depending on three-dimensional structure or on sequence. | |
===The GH32 Family=== | ===The GH32 Family=== | ||
| - | The GH32 family includes over 370 members from plants, fungi and bacteria. This family contains not only invertases but other fructofuranosidases like insulinase, transfructosidase or inulinase. | + | The GH32 family includes over 370 members from plants, fungi and bacteria. This family contains not only invertases but other fructofuranosidases like insulinase, transfructosidase or inulinase. They share a common feature which are two important amino acid residues. These two amino acid residues constitute the catalytic machinery accountable to the glycosidic bond cleavage. And they have been identified as an aspartate situated near the N-terminus and acting as the nucleophile, and a glutamate acting as the general acide/base. |
==Structure== | ==Structure== | ||
Revision as of 19:09, 31 December 2012
Please, do not delete or modify any text or image. Thank you.
Thermotoga maritima β-fructosidase (invertase)
Contents |
Introduction
The β-fructosidase (β-D-fructofuranosidase EC 3. 2. 1. 26.) of the thermophilic baterium Thermotoga maritima is an enzyme which hydrolyzes sucrose to release fructose and glucose. 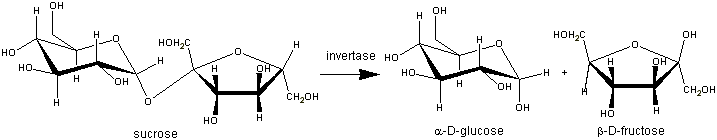
This enzyme achieves the hydrolysis with a retention molecular mechanism. Actually, the invertase hydrolyses the D-sucrose to form the D-fructose and the D-glucose. So, the substrate anomeric configuration is conserved in products. The β-fructosidase belongs to the GH32 (Glycoside hydrolysases 32) according to the sequence-based classification of glycoside hydrolysases.
Glycoside Hydrolases and the GH32 family
The Glycoside Hydrolysases
Glycoside hydrolases (GH) constitute a widespread enzyme group presenting a huge variety of protein folds and substrate specificities. They are classified into EC 3.2.1 as enzymes catalyzing the hydrolysis of O- or S-glycosides. The enzymatic hydrolysis of glycosidic bonds can be realised by two ways : inversion or retention of the product anomeric configuration compared with the substrate anomeric configuration. So we can classify Glycoside hydrolases according to these mechanismes. But we can alson make a classification depending on three-dimensional structure or on sequence.
The GH32 Family
The GH32 family includes over 370 members from plants, fungi and bacteria. This family contains not only invertases but other fructofuranosidases like insulinase, transfructosidase or inulinase. They share a common feature which are two important amino acid residues. These two amino acid residues constitute the catalytic machinery accountable to the glycosidic bond cleavage. And they have been identified as an aspartate situated near the N-terminus and acting as the nucleophile, and a glutamate acting as the general acide/base.
Structure
Active site
The β-sandwich
Applications
|
