This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 714
From Proteopedia
| Line 42: | Line 42: | ||
9/10-phosphonoxy-hydroxy-octadecanoate + H<sub>2</sub>O ↔ 9/10-dihydroxy-octadecanoate + phosphate | 9/10-phosphonoxy-hydroxy-octadecanoate + H<sub>2</sub>O ↔ 9/10-dihydroxy-octadecanoate + phosphate | ||
| - | Its <scene name='Sandbox_Reserved_714/Nter_activesite/ | + | Its <scene name='Sandbox_Reserved_714/Nter_activesite/2'>active site</scene> contains several conserved aspartates in phosphatases and phosphonatases: D9, D11, D184 and D185. This enzymatic activity is Mg<sup>2+</sup> dependant, because the structure of the active site is in its optimal conformation when the cation makes coordination interactions. When the catalytic activity of the N-term domain is available, Magnesium is octahedrally coordinated with the four aspartates, one water molecule and the phosphate belonging to the substrate. We can note that Mg<sup>2+</sup> is not directly involved in the catalytic mechanism, and all its interactions with the active site remain during the hydrolysis. Its single role consists in maintaining the three-dimensional structure of the active site. |
First, the oxygen on the lateral chain of D9 attacks the phosphate. After the addition of a proton H<sub>+</sub>, the product with its two hydroxyl functions is released, while the phosphate is still linked to D9. Then, a waters molecule binds the phosphate, breaking its bond with the aspartate. Therefore the phosphate can be finally released and the active site can accept a lipid again and start a new catalytic cycle. | First, the oxygen on the lateral chain of D9 attacks the phosphate. After the addition of a proton H<sub>+</sub>, the product with its two hydroxyl functions is released, while the phosphate is still linked to D9. Then, a waters molecule binds the phosphate, breaking its bond with the aspartate. Therefore the phosphate can be finally released and the active site can accept a lipid again and start a new catalytic cycle. | ||
Revision as of 15:40, 3 January 2013
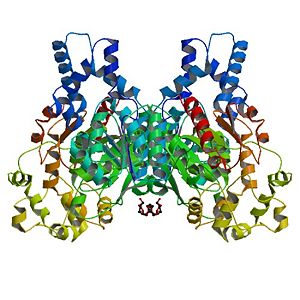
The Human soluble Epoxyde Hydroxylase (hsEH) is a homodimeric protein as well as a bifunctional enzyme, which is made of 555 aminoacids and can be found in the cytoplasm and in peroxisomes of humans. It belongs to the “Hydrolase” enzyme family. This enzyme has two functions: an epoxide hydrolase function on its C-term domain, which consists in the hydrolysis of epoxides into glycols, and a phosphatase function on its N-term domain, which consists in cleaving a phosphate group from a dihydroxy lipid phosphate.
Contents |
Biological role
This enzyme is expressed in mammalian liver, vascular endothelium, proximal tubule and some smooth muscles. By hydrolyzing epoxides and lipid phosphates, it has a role in detoxification and it prevents DNA from alkylation by those epoxides. At the physiological level, hsEH participates to the regulation of cardiovascular and renal physiology and to inflammatory biology as well[1]. More specifically, this enzyme is a regulator of mammalian blood pressure and could be a target for a treatment of hypertension[2]. Thanks to mutagenesis experiments[3], it has been demonstrated that the inhibition of sEH lowers blood pressure.
| |||||||||||
Additional 3D Structures of hsEH
1vj5 - hsEH + N-cyclohexyl-N'-(4-iodophenyl)urea complex
1zd2,1zd3, 1zd4,1zd5 - hsEH + 4-(3-cyclohexyluriedo)-carboxylic acids
3ant - Hydrolase domain + synthetic inhibitor
3pdc - Hydrolase domain + benzoxazole inhibitor
External ressources
Protein Data Bank entry on 1S8O
Uniprot link on Bifunctional epoxyde hydrolase 2
Wikipedia page on Epoxyde hydrolase 2
References
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Human soluble epoxide hydrolase: structural basis of inhibition by 4-(3-cyclohexylureido)-carboxylic acids. Protein Sci. 2006 Jan;15(1):58-64. Epub 2005 Dec 1. PMID:16322563 doi:10.1110/ps.051720206
- ↑ Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000 Dec 22;275(51):40504-10. PMID:11001943 doi:10.1074/jbc.M008106200
- ↑ Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002 Feb;39(2 Pt 2):690-4. PMID:11882632
- ↑ Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311-33. PMID:15822179 doi:10.1146/annurev.pharmtox.45.120403.095920
- ↑ Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004 Apr 27;43(16):4716-23. PMID:15096040 doi:10.1021/bi036189j
- ↑ Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1558-63. Epub 2003 Feb 6. PMID:12574510 doi:10.1073/pnas.0437724100
Proteopedia Page Contributors and Editors
DUTREUX Fabien, BONHOURE Anna

