Sandbox Reserved 709
From Proteopedia
(→Cytoplasm to nucleus) |
m (Added scheme v2 (Nup is a nucleoporin)) |
||
| Line 5: | Line 5: | ||
==Nuclear import of proteins== | ==Nuclear import of proteins== | ||
===Cytoplasm to nucleus=== | ===Cytoplasm to nucleus=== | ||
| - | [[Image: | + | [[Image:Nuclear_import_protein.png | 600px | right |thumb]] |
In eukaryotic cells, a lot of proteins are selectively imported from the cytosol to the nucleus. These proteins have to pass through '''NPC'''s ('''Nuclear Pore Complexes'''). | In eukaryotic cells, a lot of proteins are selectively imported from the cytosol to the nucleus. These proteins have to pass through '''NPC'''s ('''Nuclear Pore Complexes'''). | ||
Revision as of 20:45, 3 January 2013
Contents |
Nuclear import of proteins
Cytoplasm to nucleus
In eukaryotic cells, a lot of proteins are selectively imported from the cytosol to the nucleus. These proteins have to pass through NPCs (Nuclear Pore Complexes).
NPCs are composed of 30 nucleoporins and weight 125 MDa. Because of their particular shape, diffusion will take more time for a bigger protein. Thus, proteins over 60 kDa have trouble to pass through NPCs that’s why they need complementary proteins (importins and exportins).
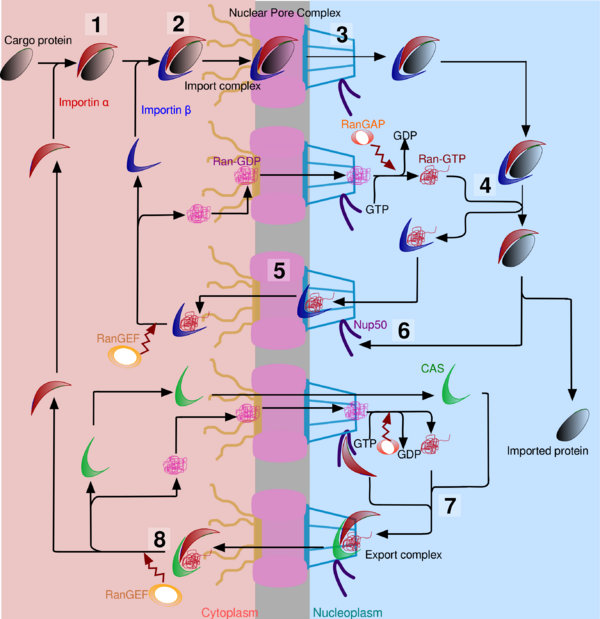
These proteins which have to go in the nucleus possess a NLS (Nuclear Localization Signal) which is responsible for the selectivity of the active transport of proteins. NLS can be found at any place of the amino acid sequence and often have a loop structure at the surface of proteins. The transport through NPC is not the same as transmembrane transport in the organelles because here, it is an aquifer pore whereas transmembrane transport in organelles involves transmembrane proteins. Thus, fully folded nuclear proteins can pass through NPC. Nevertheless, it seems that really large proteins undergo a compression when they pass through NPC. To initiate the transport to the nucleus, most of the NLS-containing proteins (or cargos) have to be recognized by Nuclear Import Receptors. These are soluble cytosolic proteins such as importin α and importin β. Import through NPC follows different steps :
- The NLS of the protein binds to the NLS-binding site of importin α.
- Importin α binds to importin β because of its importin β binding domain (IBB)
- Importin β binds to some fibrils of the NPC which contain a lot of short amino-acid repeats that contain phenylalanine and glycine and are therefore called FG-repeats.
- The complex cargo:importin α:importin β move along the NPC by repeatedly binding, dissociating and re-binding to adjacent FG-repeat sequences.
Cargo release and protein recycling
Once the import complex (made of importin α, importin β and cargo protein) enters the nucleus, it must be dissociated to release the cargo protein. Then, importin α must be recycled to the cytoplasm. Cargo release is driven by Ran, a G-protein that is found in Ran-GTP form in the nucleus due to a high rate of Ran-GEF. It binds to importin β, causing an important change in shape. An other protein, Nup50 (also called Npap60), weakens the link between importin α and the NLS of the cargo. Thus the cargo protein is now free in the nucleoplasm. Importin β can pass through the NPC thanks to Ran-GTP, and once it is in the cytoplasm, it is released by the hydrolysis of Ran-GTP into Ran-GDP due to a higher rate of Ran-GAP in the cytoplasm. But importin α can’t leave the nucleus only with the help of Ran-GTP. It needs also interaction with CAS (Cellular Apoptosis Susceptibility protein), an exportin which is similar in shape to importin β. Binding between CAS and importin α is favoured by interactions with Nup50. The newly formed importin α, CAS and Ran-GTP complex is able to interact with nucleoplasmins of NPC, and once in the cytoplasm, Ran-GTP is hydrolysed into Ran-GDP. The complex splits, and importin α is now ready for a new import cycle.
Importin α structure
|
Importin α is a soluble adaptor protein also known as karyopherin α. Its function is to bind a protein containing a cNLS (classical Nuclear Localization Signal) and then to bind an importin β in order to help the import of this protein in the nucleus.
A cNLS is a basic residue-rich sequence with the following consensus sequence :
- Two adjacent basic amino acids (Arg or Lys).
- A spacer region of 10 residues.
- At least three basic residues (Arg or Lys) in the five positions after the spacer region.
Importin α is composed of different domains:
- A flexible and hydrophilic 10kDa N-terminal Importin β binding domain (IBB domain). The IBB domain is a L-shapped molecule with an N-terminal extended moiety and a C-terminal helix running in mutually perpendicular directions. Because this domain is highly positively charged, it can binds to the inner surface of importin-β that contains many acidic residues. It has been shown that importin α contains a determinant which is sufficient for binding importin β. The consensus sequence of this determinant is "KFRLLSKE". The serine contained in this sequence is present in all importin α which shows its importance. However, the upstream region is sufficient for binding importin β too. Nowadays, we think that this upstream region contribute to the strength of the bond. This could explain the fact that the binding between importin α and β is stronger when α contains these two determinants.
- A 50kDa C-terminal NLS-binding site composed of 10 tandem armadillo (Arm) repeats. These arm repeat domains have an elongated superhelical structure and each of them contains 3 α-helices (H1, H2, H3). Together, H3 helices define the inner concave surface of the protein and the NLS-binding site.
- A NLS. Thus, importin α belongs to the group of proteins containing both a ligand (NLS) and a cognate receptor (NLS-binding site). That’s why it could have a possibility of autologous ligand-receptor interactions. Nevertheless, it has been shown that NLS of importin α overlaps with the IBB. Thereby, binding of importin β to importin α covers the NLS of importin α preventing autologous ligand receptor interactions.
- A CAS-binding site. CAS or cellular apoptosis susceptibility protein is an exportin which in the nucleus is bound to RanGTP.
Importin α : Nup50 complex
|
and
See Also
Reference
- Matsuura Y, Stewart M. Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling. EMBO J. 2005 Nov 2;24(21):3681-9. Epub 2005 Oct 13. PMID:16222336