Group:MUZIC:Myozenin
From Proteopedia

(→Sequence Annotation) |
(→Function and Interactions) |
||
| Line 11: | Line 11: | ||
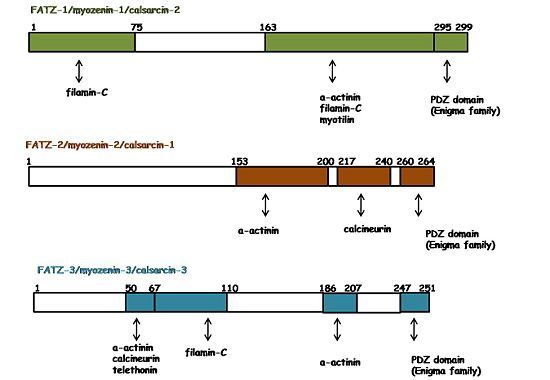
[[Image:FATZ_binding_map.jpg|550px|left|thumb|Binding regions on FATZ proteins]]The three protein members of the FATZ family have a plethora of interacting partners which are additionally shared by all of them. As shown by the figure on the right, they interact with several Z-disc proteins. For instance: α-actinin-2, filamin-C, myotilin, telethonin, calcineurin and ZASP/Cypher (in general the Enigma protein family) <ref name="r2">PMID: 11171996</ref><ref name="r4">PMID: 11842093</ref><ref>PMID: 19047374</ref><ref>PMID: 16076904</ref><ref name="r3">PMID: 11114196</ref><ref name="r1">PMID: 10984498</ref>. In general, those interactions and their binding regions were found by yeast two-hybrid assays, co-immunoprecipitation, and pull down assays. | [[Image:FATZ_binding_map.jpg|550px|left|thumb|Binding regions on FATZ proteins]]The three protein members of the FATZ family have a plethora of interacting partners which are additionally shared by all of them. As shown by the figure on the right, they interact with several Z-disc proteins. For instance: α-actinin-2, filamin-C, myotilin, telethonin, calcineurin and ZASP/Cypher (in general the Enigma protein family) <ref name="r2">PMID: 11171996</ref><ref name="r4">PMID: 11842093</ref><ref>PMID: 19047374</ref><ref>PMID: 16076904</ref><ref name="r3">PMID: 11114196</ref><ref name="r1">PMID: 10984498</ref>. In general, those interactions and their binding regions were found by yeast two-hybrid assays, co-immunoprecipitation, and pull down assays. | ||
| - | The interaction of FATZ-1 with α-actinin-2 has been reported on the CD2 domain of all FATZ proteins<ref name="r1">PMID: 10984498</ref><ref name="r2">PMID: 11171996 </ref><ref name="r4">PMID: 11842093</ref>. | + | The interaction of FATZ-1 with α-actinin-2 has been reported on the CD2 domain of all FATZ proteins<ref name="r1">PMID: 10984498</ref><ref name="r2">PMID: 11171996 </ref><ref name="r4">PMID: 11842093</ref>. The data suggest a binding interface on α-actinin-2 spanning the spectrin repeat domains SR2,SR3 and SR4. FATZ-1, α-actinin-2 and myotilin appear in premyofibrils, when there is no Z-disc but a small structure called Z-body. In addition, the complexes FATZ-1::α-actinin-2, FATZ-1::myotilin and myotilin::α-actinin-2 are observed in that early stages. In contrast, telethonin localized to the Z-disc in later stages and the complex FATZ-1::telethonin was only observed in mature myofibrils. These findings suggest that the interactions of FATZ-1 with α-actinin-2 and myotilin are very important for the initiation of the Z-disc assembly. Besides, it is hypothesized that FATZ-1 should undergone conformational changes during myofibrilogenesis to interact with telethonin. Thus, telethonin incorporation to the the Z-disc depends to FATZ-1 viability <ref> PMID: 15810059 </ref> . It is suggested that FATZ-1 performs other functions, like bridging filamin-C and α-actinin-2. However, a competitive binding assay showed that α-actinin-2 displaces filamin-C from FATZ-1. Therefore, it still remains an open question whether a ternary complex could exist and what its physiological role would be <ref name="r2">PMID: 11171996</ref>. |
| - | Both proteins, FATZ-1 and FATZ-2 | + | Both proteins, FATZ-1 and FATZ-2 are negative regulators of the calcineurin/NFAT pathway in striated muscle. The activation of the calcineurin/NFAT pathway determines the switch to slow-twitch fiber phenotype in skeletal muscle. The isoform FATZ-1 (Calsarcin-2 and Myozenin-1) is a negative regulator of the Calcineurin/NFAT pathway in fast fibers. In absence of FATZ-1 the activity of the Calcineurin/NFAT signaling pathway increases, consequently, there are a major number of oxidative fibers, and less fatigue of FATZ-1 knockdown compared to FATZ-1 wild type mice subjected to long endurance exercise. The interaction between FATZ-1 and Calcineurin is another check point in the signaling pathways controlling skeletal muscle fiber type composition and response to exercise performance <ref>PMID: 2564612</ref>. FATZ-2 is a negative regulator of Calcineurin in cardiac muscle, leading to cardiac hypertrophic phenotype in FATZ-2 deficient mouse subjected to hypertrophic agonists <ref>PMID: 15543153</ref>. |
== Pathology== | == Pathology== | ||
Revision as of 22:22, 5 February 2013
Contents |
Introduction
The filamin-C α-actinin telethonin Z-disc binding protein (FATZ) is a protein family of three members: FATZ-1, FATZ-2, FATZ-3, which are expressed in muscle cells[1] This protein family also known as Myozenin or Calsarcin, is mainly localized in the Z-disc, although recently it has been described that FATZ-2 appears in cardiac nuclei[2]. The expression of all three proteins has been shown to be fibre type specific. For instance, FATZ-1 and FATZ-3 are exclusively expressed in skeletal muscle fast-twitch fibres while FATZ-2 is expressed in cardiac and slow-twitch fibres[3][4]. FATZ proteins have multiple binding partners in the Z-disc, which involve them in different functions like the Z-disc formation and maintenance or in signaling pathways like the calcineurin/NFAT[5]. Therefore, the FATZ protein family could be seen as one example of Z-disc proteins where signalling and structural support converge.
Sequence Annotation
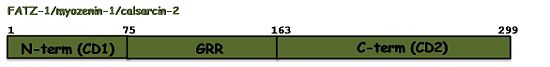
The sequence annotation of FATZ-1 is related to the sequence conservation profile among the three isoforms, which share well conserved N-terminal and C-terminal regions. Therefore, the N-terminal of FATZ-1 was named conserved domain 1 (CD1, 1-75aa) and its C-terminal conserved domain 2 (CD2, 163-299aa). Both regions are connected by a stretch of amino acids with a 39.5% of glycine. Consequently, this region was named as glycine rich region (GRR, 75-162aa)[6] Q9NP98. Although the N-terminal and C-terminal regions of the other two proteins could be also named CD1 and CD2 no such sequence annotations exist in their UniProtKB entries Q9NPC6, Q8TDC0.Function and Interactions
The three protein members of the FATZ family have a plethora of interacting partners which are additionally shared by all of them. As shown by the figure on the right, they interact with several Z-disc proteins. For instance: α-actinin-2, filamin-C, myotilin, telethonin, calcineurin and ZASP/Cypher (in general the Enigma protein family) [6][4][7][8][3][1]. In general, those interactions and their binding regions were found by yeast two-hybrid assays, co-immunoprecipitation, and pull down assays.The interaction of FATZ-1 with α-actinin-2 has been reported on the CD2 domain of all FATZ proteins[1][6][4]. The data suggest a binding interface on α-actinin-2 spanning the spectrin repeat domains SR2,SR3 and SR4. FATZ-1, α-actinin-2 and myotilin appear in premyofibrils, when there is no Z-disc but a small structure called Z-body. In addition, the complexes FATZ-1::α-actinin-2, FATZ-1::myotilin and myotilin::α-actinin-2 are observed in that early stages. In contrast, telethonin localized to the Z-disc in later stages and the complex FATZ-1::telethonin was only observed in mature myofibrils. These findings suggest that the interactions of FATZ-1 with α-actinin-2 and myotilin are very important for the initiation of the Z-disc assembly. Besides, it is hypothesized that FATZ-1 should undergone conformational changes during myofibrilogenesis to interact with telethonin. Thus, telethonin incorporation to the the Z-disc depends to FATZ-1 viability [9] . It is suggested that FATZ-1 performs other functions, like bridging filamin-C and α-actinin-2. However, a competitive binding assay showed that α-actinin-2 displaces filamin-C from FATZ-1. Therefore, it still remains an open question whether a ternary complex could exist and what its physiological role would be [6].
Both proteins, FATZ-1 and FATZ-2 are negative regulators of the calcineurin/NFAT pathway in striated muscle. The activation of the calcineurin/NFAT pathway determines the switch to slow-twitch fiber phenotype in skeletal muscle. The isoform FATZ-1 (Calsarcin-2 and Myozenin-1) is a negative regulator of the Calcineurin/NFAT pathway in fast fibers. In absence of FATZ-1 the activity of the Calcineurin/NFAT signaling pathway increases, consequently, there are a major number of oxidative fibers, and less fatigue of FATZ-1 knockdown compared to FATZ-1 wild type mice subjected to long endurance exercise. The interaction between FATZ-1 and Calcineurin is another check point in the signaling pathways controlling skeletal muscle fiber type composition and response to exercise performance [10]. FATZ-2 is a negative regulator of Calcineurin in cardiac muscle, leading to cardiac hypertrophic phenotype in FATZ-2 deficient mouse subjected to hypertrophic agonists [11].
Pathology
It was shown that FATZ-2 KO mouse hearts subjected to pressure overload chronically activated the calcineurin/NFAT signalling pathway. In consequence, those animals showed hypertrophic hearts and developed cardiomyopathy. Moreover, the transgenic over-expression of FATZ-2 rescued the mouse hearts from angiotensin II-induced cardiac hypertrophy, which suggested that FATZ-2 could be a target gene affected in patients with the same disease[5]. Later on, the investigation of two families with clinical symptoms of hypertrophic cardiomyopathy(HCM) suggested that mutations S48P and I246M in FATZ-2 where associated with familial HCM[12]. However, another study on 438 patiens concluded that mutations in FATZ-2 were rare causes of familial HCM [13]. Recently, a set of experiments in mice models expressing the mutants FATZ-2 S48P and I246M showed the clinical symptoms of HCM, myofibrillar disarray and Z-disc structural abnormalities. However, no upregulation of the calcineurin/NFAT signalling pathway was observed, suggesting that HCM caused by those mutations is not associated to the inhibitory effect of FATZ-2 on calcineurin [14].
References
- ↑ 1.0 1.1 1.2 Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000 Dec 29;275(52):41234-42. PMID:10984498 doi:10.1074/jbc.M007493200
- ↑ Paulsson AK, Franklin S, Mitchell-Jordan SA, Ren S, Wang Y, Vondriska TM. Post-translational regulation of calsarcin-1 during pressure overload-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010 Jun;48(6):1206-14. Epub 2010 Feb 17. PMID:20170660 doi:10.1016/j.yjmcc.2010.02.009
- ↑ 3.0 3.1 Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14632-7. PMID:11114196 doi:10.1073/pnas.260501097
- ↑ 4.0 4.1 4.2 Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002 Apr 19;277(16):13998-4004. Epub 2002 Feb 12. PMID:11842093 doi:10.1074/jbc.M200712200
- ↑ 5.0 5.1 Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004 Dec;10(12):1336-43. Epub 2004 Nov 14. PMID:15543153 doi:nm1132
- ↑ 6.0 6.1 6.2 6.3 Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1595-600. Epub 2001 Feb 6. PMID:11171996 doi:10.1073/pnas.041609698
- ↑ von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-disc myopathies. Mol Cell Biol. 2009 Feb;29(3):822-34. Epub 2008 Dec 1. PMID:19047374 doi:10.1128/MCB.01454-08
- ↑ Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, Faulkner G, Borradori L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005 Aug 15;118(Pt 16):3739-49. Epub 2005 Aug 2. PMID:16076904 doi:10.1242/jcs.02484
- ↑ Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005 May;61(1):34-48. PMID:15810059 doi:10.1002/cm.20063
- ↑ Conlon KC, Bading JR, DiResta GR, Corbally MT, Gelbard AS, Brennan MF. Validation of transport measurements in skeletal muscle with N-13 amino acids using a rabbit isolated hindlimb model. Life Sci. 1989;44(13):847-59. PMID:2564612
- ↑ Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004 Dec;10(12):1336-43. Epub 2004 Nov 14. PMID:15543153 doi:nm1132
- ↑ Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, Roberts R, Willerson JT, Marian AJ. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007 Mar 30;100(6):766-8. Epub 2007 Mar 8. PMID:17347475 doi:10.1161/01.RES.0000263008.66799.aa
- ↑ Posch MG, Thiemann L, Tomasov P, Veselka J, Cardim N, Garcia-Castro M, Coto E, Perrot A, Geier C, Dietz R, Haverkamp W, Ozcelik C. Sequence analysis of myozenin 2 in 438 European patients with familial hypertrophic cardiomyopathy. Med Sci Monit. 2008 Jul;14(7):CR372-4. PMID:18591919
- ↑ Ruggiero A, Chen SN, Lombardi R, Rodriguez G, Marian AJ. Pathogenesis of hypertrophic cardiomyopathy caused by myozenin 2 mutations is independent of calcineurin activity. Cardiovasc Res. 2012 Oct 19. PMID:22987565 doi:10.1093/cvr/cvs294