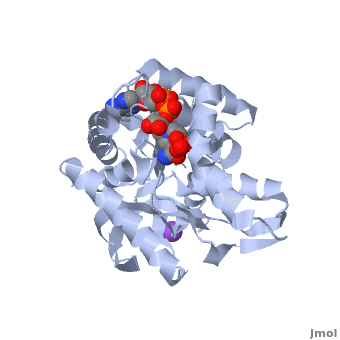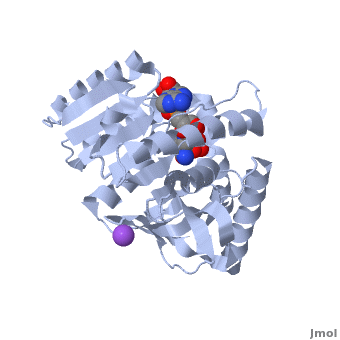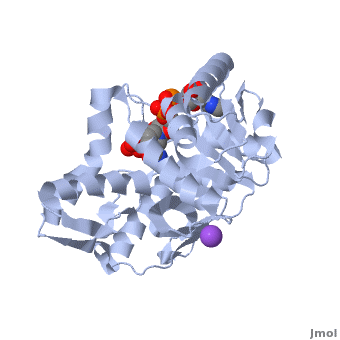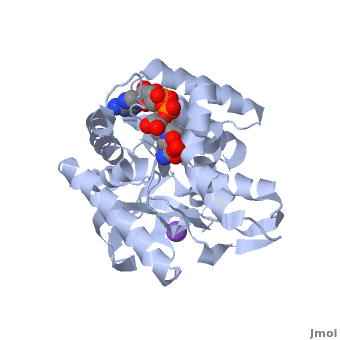
Structure
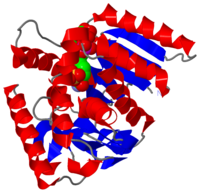
The secondary structure of a single subunit contains a wrapped by . Near the sodium bound end, 4 small anti-parallel beta sheets and 1 small alpha helix enable a turn in the residue chain. Opposite the sodium bound ligand, 6 alpha helices point towards a common point, three on each side of the beta sheet backbone. The alpha helices form a for a NAD+ cofactor to attach near the beta sheeting. The structure most nearly resembles an alternating alpha/beta classification. As for the 3D structure, the enzyme forms a sort of
for the substrate to bind.
Mechanism
The mechanism of catalysis is dependent on . These residues are HIS 195 and ASP 168, which are both involved in hydrogen bonding, ASP 53 associated with , and a triad of arginine residues at 102, 109, and 171. During the conversion of malate to oxaloacetate, a key conformational change occurs on the binding of substrate in which a “loop” flips into an up position to block the active site from the solvent.
 When this occurs, the other residues in the active site are brought closer to the substrate to enable the conversion. R102 and R109 are involved in this loop flip and thus invariant. After the loop flip, the malate complex is stabilized via hydrogen bonding before accepting a proton transfer from NADH to form oxaloacetate [7].
When this occurs, the other residues in the active site are brought closer to the substrate to enable the conversion. R102 and R109 are involved in this loop flip and thus invariant. After the loop flip, the malate complex is stabilized via hydrogen bonding before accepting a proton transfer from NADH to form oxaloacetate [7].
Evolutionary Divergence
The evolutionary past of MDH shows a divergence to form lactate dehydrogenase (LDH) which functions in a very similar way to MDH. Although there is a very low sequence conservation among MDH and LDH’s [1] the structure of the enzyme has remained relatively conserved. The key difference between the two is in the substrate: LDH catalyzes pyruvate to lactate.
3D Structures of Malate Dehydrogenase
Update February 2013
The holo-MDH contains NAD or its derivatives while the apo-MDH lacks it.
Holo-MDH
2x0r – HmMDH (mutant)+NAD - Haloarcula marismortui
1o6z - HmMDH (mutant)+NADH
1hlp – HmMDH+NAD
1x0i – AfMDH+NADH – Archaeoglobus fulgidus
2x0j - AfMDH+etheno-NAD
1hlp – HmMDH+NAD
1x0i – AfMDH+NADH
2x0j - AfMDH+etheno-NAD
1ib6, 1ie3 – EcMDH (mutant)+NAD - Escherichia coli
1emd – EcMDH+NAD+citrate
3i0p – MDH+NAD – Entamoeba histolytica
3gvh – BmMDH+NAD – Brucella melitensis
3gvi - BmMDH+ADP
2hjr – MDH+adenosine diphosphoribose – Cryptosporidium parvum
2dfd – MDH+NAD – human type 2
1wze – TfMDH (mutant)+NAD – Thermus flavus
1wzi - TfMDH (mutant)+NDP
1bdm - TfMDH (mutant)+beta-6-hydroxy-1,4,5,6-tetrahydronicotinamide adenine dinucleotide
1bmd – TfMDH+NAD
1y7t – TtMDH+NADPH – Thermus thermophilus
2cvq - TtMDH+NADP
1v9n – MDH+NADPH – Pyrococcus horikoshii
1z2i – MDH+NAD – Agrobacterium tumefaciens
1uxg, 1uxh, 1uxi, 1uxj, 1uxk, 1ur5 – MDH (mutant)+NAD – Chloroflexus aurantiacus
1guz, 1guy, 1gv0 – CvMDH+NAD – Chlorobium vibrioforme
1civ – MDH+NADP – Flaveria bidentis
1b8u, 1b8v – AaMDH+NAD - Aquaspirillum arcticum
5mdh – SsMDH+NAD+alpha-ketomalonic acid – Sus scrofa
4mdh – SsMDH+NAD
4i1i – LmMd + NAD – Leishmania major
apo-MDH
2j5r, 2j5k, 2j5q, 1d3a – HmMDH
2hlp – HmMDH (mutant)
3hhp, 2pwz – EcMDH
3fi9 – MDH – Porphyromonas gingivalis
3d5t - MDH – Burkholderia pseudomallei
2d4a – MDH – Aeropyrum pernix
1iz9 - TtMDH
1sev, 1smk – MDH – Citrullus lanatus
1gv1 – CvMDH
1b8p – AaMDH
7mdh – MDH – Sorgum bicolor
1mld – SsMDH
2cmd - EcMd+citrate
3nep – Md – Salinibacter ruber
3p7m – Md – Francisella tularensis
3tl2 – Md – Bacillus anthracis
4e0b – Md – Vibrio vulnificus
4h7p - LmMd
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Minarik P, Tomaskova N, Kollarova M, Antalik M. Malate dehydrogenases--structure and function. Gen Physiol Biophys. 2002 Sep;21(3):257-65. PMID:12537350
- ↑ Matsuda T, Takahashi-Yanaga F, Yoshihara T, Maenaka K, Watanabe Y, Miwa Y, Morimoto S, Kubohara Y, Hirata M, Sasaguri T. Dictyostelium Differentiation-Inducing Factor-1 Binds to Mitochondrial Malate Dehydrogenase and Inhibits Its Activity. J Pharmacol Sci. 2010 Feb 20. PMID:20173310
- ↑ Matsuda T, Takahashi-Yanaga F, Yoshihara T, Maenaka K, Watanabe Y, Miwa Y, Morimoto S, Kubohara Y, Hirata M, Sasaguri T. Dictyostelium Differentiation-Inducing Factor-1 Binds to Mitochondrial Malate Dehydrogenase and Inhibits Its Activity. J Pharmacol Sci. 2010 Feb 20. PMID:20173310
- ↑ Musrati RA, Kollarova M, Mernik N, Mikulasova D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998 Sep;17(3):193-210. PMID:9834842
- ↑ Boernke WE, Millard CS, Stevens PW, Kakar SN, Stevens FJ, Donnelly MI. Stringency of substrate specificity of Escherichia coli malate dehydrogenase. Arch Biochem Biophys. 1995 Sep 10;322(1):43-52. PMID:7574693 doi:http://dx.doi.org/10.1006/abbi.1995.1434
- ↑ Plancarte A, Nava G, Mendoza-Hernandez G. Purification, properties, and kinetic studies of cytoplasmic malate dehydrogenase from Taenia solium cysticerci. Parasitol Res. 2009 Jul;105(1):175-83. Epub 2009 Mar 10. PMID:19277715 doi:10.1007/s00436-009-1380-6
- ↑ Goward CR, Nicholls DJ. Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci. 1994 Oct;3(10):1883-8. PMID:7849603 doi:http://dx.doi.org/10.1002/pro.5560031027