Structural templates
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | ==Motifs In Proteins== | ||
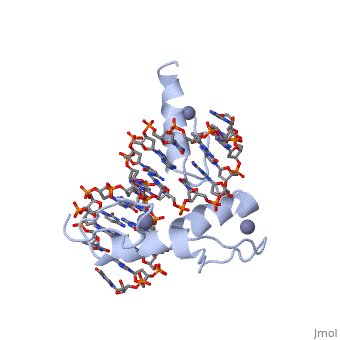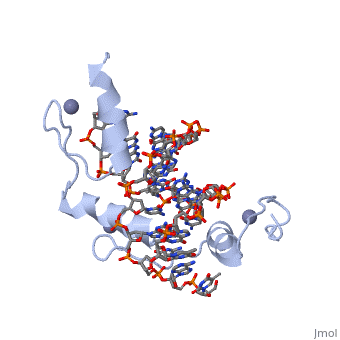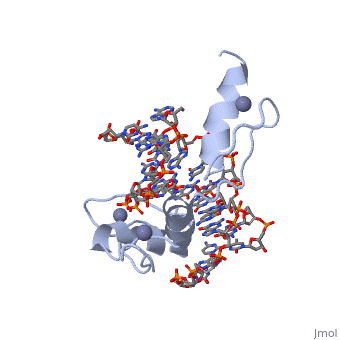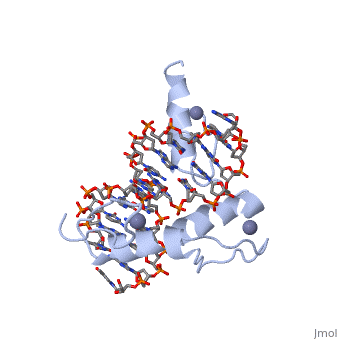
<StructureSection load='1aay' size='350' side='right' scene='' caption=''> | <StructureSection load='1aay' size='350' side='right' scene='' caption=''> | ||
| + | ==Motifs In Proteins== | ||
The term "motif" when used in structural biology tends to refer to one of two cases: | The term "motif" when used in structural biology tends to refer to one of two cases: | ||
<OL> | <OL> | ||
| Line 60: | Line 60: | ||
===β-sheets=== | ===β-sheets=== | ||
| - | < | + | |
| - | + | In the <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_start/2'>Jmol viewer to the right PDB entry 5p21</scene> has been coloured by secondary structure (α-helices are coloured magenta and β-strands are coloured yellow). A single <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_beta/1'>beta-strand</scene> can technically be described as a flat helix with 2 residues per turn although this may not be initially obvious. <br> | |
When two or more beta strands lie next to each other, forming hydrogen bonds between them, this is what is termed a <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_betasheet/1'>β-sheet</scene>. As the backbones need to come close together to interact and form a sheet, the <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_beta_sc/1'>sidechains are oriented away from the plane of the sheet</scene>. As the polypeptide chain is synthesised from the amino terminus to the carboxyl terminus it has a directionality (represented in the cartoon format as an arrowhead on each beta strand). β-sheets therefore occur in two varieties:<OL> | When two or more beta strands lie next to each other, forming hydrogen bonds between them, this is what is termed a <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_betasheet/1'>β-sheet</scene>. As the backbones need to come close together to interact and form a sheet, the <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_beta_sc/1'>sidechains are oriented away from the plane of the sheet</scene>. As the polypeptide chain is synthesised from the amino terminus to the carboxyl terminus it has a directionality (represented in the cartoon format as an arrowhead on each beta strand). β-sheets therefore occur in two varieties:<OL> | ||
<LI><scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_beta_ap/1'>Anti-parallel</scene> - here the beta strands aligned next to each other run in opposite directions. As the interacting carbonyls and amides align well, the hydrogen bonds appear to be straight. | <LI><scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_beta_ap/1'>Anti-parallel</scene> - here the beta strands aligned next to each other run in opposite directions. As the interacting carbonyls and amides align well, the hydrogen bonds appear to be straight. | ||
| Line 69: | Line 69: | ||
===Turns and loops=== | ===Turns and loops=== | ||
| - | + | ||
There are a number of small hydrogen bonded motifs and patterns which are observed regularly. These are described below:<UL> | There are a number of small hydrogen bonded motifs and patterns which are observed regularly. These are described below:<UL> | ||
<LI>'''<scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_betaturn/1'>Beta Turns</scene>''' - originally defined by the one hydrogen bond common to all (an i, i+3 hydrogen bond) but some modern descriptions do not require a hydrogen bond. | <LI>'''<scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_betaturn/1'>Beta Turns</scene>''' - originally defined by the one hydrogen bond common to all (an i, i+3 hydrogen bond) but some modern descriptions do not require a hydrogen bond. | ||
| Line 81: | Line 81: | ||
<br> | <br> | ||
{{Clear}} | {{Clear}} | ||
| - | + | ||
These secondary structure motifs can be combined to form functional motifs, the most well known of which is the helix-turn-helix motif found in a number of DNA-binding proteins. The computational identification of these motifs is straightforward but made complicated by the fact that not all helix-turn-helix motifs bind DNA. The problem faced here is therefore one involving the distinguishing between true and false positives. The structure to the right is that of lambda repressor bound to DNA. The helix-turn-helix motif is readily identified in green. | These secondary structure motifs can be combined to form functional motifs, the most well known of which is the helix-turn-helix motif found in a number of DNA-binding proteins. The computational identification of these motifs is straightforward but made complicated by the fact that not all helix-turn-helix motifs bind DNA. The problem faced here is therefore one involving the distinguishing between true and false positives. The structure to the right is that of lambda repressor bound to DNA. The helix-turn-helix motif is readily identified in green. | ||
| Line 98: | Line 98: | ||
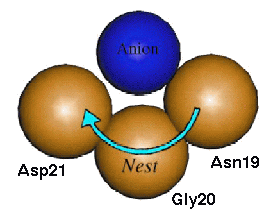
In compound nests the result is a long chain with all the overlapping nests facing a similar direction. This basically forms a much wider nest that is capable of binding a larger anionic group of atoms such as the phosphate ion, and are usually functionally important motifs. Tandem nests are not as common and, due to the greater change in the direction that adjacent nests face, only seem to perform functional roles when found in conjunction with one or more compound nests. | In compound nests the result is a long chain with all the overlapping nests facing a similar direction. This basically forms a much wider nest that is capable of binding a larger anionic group of atoms such as the phosphate ion, and are usually functionally important motifs. Tandem nests are not as common and, due to the greater change in the direction that adjacent nests face, only seem to perform functional roles when found in conjunction with one or more compound nests. | ||
| - | <applet load='1lmb' size='300' frame='true' align='left' caption='Nest in PDB entry 5p21' scene='User:James_D_Watson/Structural_Templates/Secondary_structure_start/4'/> | ||
One of the most well known functional compound nests is found in the phosphate-binding loop of Ras protein (PDB entry 5p21). The <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_ploop/1'>P-loop</scene> is a well described ATP- or GTP-binding loop present in a large superfamily of important proteins which includes G-proteins and kinases. The main feature of the P-loop is a long compound LRLR nest that <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_ploop_nest/1'>forms a binding site for the β-phosphate of ATP or GTP</scene>. However, this is an example of a motif where the ligand also binds to the free main chain NH groups at the N-terminus of an alpha helix. On closer inspection it becomes evident that this interaction is in addition to the compound nest and does not interfere with it. Therefore the P-loop is actually more accurately described as a compound LRLR nest and an adjacent helical N-terminus that collectively bind to the α- and β-phosphates of the GDP substrate. The P-loop, which is retained throughout the superfamily, has a highly conserved GxxxxGKS/T consensus sequence (where the xxGK section forms the LRLR compound nest). | One of the most well known functional compound nests is found in the phosphate-binding loop of Ras protein (PDB entry 5p21). The <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_ploop/1'>P-loop</scene> is a well described ATP- or GTP-binding loop present in a large superfamily of important proteins which includes G-proteins and kinases. The main feature of the P-loop is a long compound LRLR nest that <scene name='User:James_D_Watson/Structural_Templates/Secondary_structure_ploop_nest/1'>forms a binding site for the β-phosphate of ATP or GTP</scene>. However, this is an example of a motif where the ligand also binds to the free main chain NH groups at the N-terminus of an alpha helix. On closer inspection it becomes evident that this interaction is in addition to the compound nest and does not interfere with it. Therefore the P-loop is actually more accurately described as a compound LRLR nest and an adjacent helical N-terminus that collectively bind to the α- and β-phosphates of the GDP substrate. The P-loop, which is retained throughout the superfamily, has a highly conserved GxxxxGKS/T consensus sequence (where the xxGK section forms the LRLR compound nest). | ||
Revision as of 10:10, 11 March 2013
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, James D Watson, Jaime Prilusky, Eran Hodis