We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hexoses
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
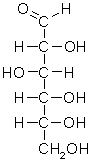
== Fructose == | == Fructose == | ||
| - | The applet on the right shows <scene name='Hexoses/Open_fructose/3'>D-fructose</scene> in a conformation in which the oxygen of C-5 is in position to react with C-2, the carbonyl carbon, forming a hemiketal<ref>[http://en.wikipedia.org/wiki/Hemiacetal Hemiketal]</ref>. As in the case of glucose forming a hemiacetal, the carbonyl carbon becomes a chiral carbon and an anomeric carbon. The two possible anomers are called <scene name='Hexoses/Alpha_fructose/3'>α-D-fructofuranose</scene> <ref>[http://en.wikipedia.org/wiki/Furanose Furanose]</ref> and <scene name='Hexoses/Beta_fructose/2'>β-D- fructofuranose</scene> | + | The applet on the right shows <scene name='Hexoses/Open_fructose/3'>D-fructose</scene> in a conformation in which the oxygen of C-5 is in position to react with C-2, the carbonyl carbon, forming a hemiketal<ref>[http://en.wikipedia.org/wiki/Hemiacetal Hemiketal]</ref>. As in the case of glucose forming a hemiacetal, the carbonyl carbon becomes a chiral carbon and an anomeric carbon. The two possible anomers are called <scene name='Hexoses/Alpha_fructose/3'>α-D-fructofuranose</scene> <ref>[http://en.wikipedia.org/wiki/Furanose Furanose]</ref> and <scene name='Hexoses/Beta_fructose/2'>β-D- fructofuranose</scene>. |
| - | The α anomer | + | The α anomer is shown with an edge-on-view, with the anomeric carbon (C-2) on the right side of the structure and with its hydroxyl group projecting down. C-1 is not part of the five membered ring and projects above the ring. |
| - | + | ||
| - | + | ||
== Terms Defined in Wikipedia == | == Terms Defined in Wikipedia == | ||
Revision as of 14:14, 20 March 2013
| |||||||||||