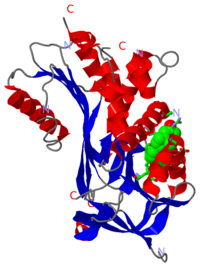
Crystal Structure of corticosteroid-binding globulin complex with cortisol,
2v95 Introduction
Corticosteroid-binding globulin (CBG) is a blood plasma protein 50 to 60 kDa in size [1],[2]. Although it is a member of the serine protease inhibitor (SERPIN) structural family, it does not have any known intrinsic serine protease inhibitor activity [3]. Its physiological function is conventionally thought to be the transport of the weakly water-soluble hormone, cortisol, throughout the circulation [4],[5]. Structural studies of native rat CBG [6] and a cleaved human CBG-antitrypsin chimera [7] have corroborated earlier biochemical studies showing that cortisol binds to CBG with a one-to-one stoichiometry. Additionally, CBG is believed to buffer the concentration of free cortisol in the blood, keeping the level constant despite its pulsatile secretion pattern [8]. CBG is also thought to participate in the stress response by releasing cortisol specifically at inflammatory sites upon cleavage by human neutrophil elastase between residues 344 and 345[9].
Gene
The gene encoding corticosteroid-binding globulin is located at the 370kb long chromosome locus 14q32.1 among ten other related genes and pseudogenes. The transcriptional activation of this gene cluster appears to be coordinately regulated by a locus control region upstream of the alpha-1-anti-trypsin gene that responds to transcription factors such as hepatic nuclear factors HNF-1 and -4[10],[11].
Structure
Corticosteroid-binding globulin is a globular protein comprising mainly of alpha-helices and beta-sheets. There are and in its tertiary structure, as shown in the interactive diagram displayed here.
Previously-solved structures of the human CBG-antitrypsin (Pittsburgh) chimera with cleaved reactive centre loop at 1.84Å and the native rat CBG at 1.9Å confirm that corticosteroid-binding globulin, despite being a non-inhibitory member of the serpin family, undergoes the S-to-R transition just like the inhibitory members [12],[13].
In its native form, CBG is in the "stressed" conformation and binds cortisol with very high affinity[14].
Upon cleavage by its target proteinase, believed to be human neutrophil elastase, the protein undergoes structural rearrangements in the main chain at several parts of the serpin fold, resulting in what is conventionally known as the "relaxed" state [15],[16], as seen . The main conformational change is the insertion of the entire N-terminal segment of the reactive loop into beta-sheet A where it is incorporated as a .
(Click to return to structure of CBG in the S-state.)
Ligand-binding
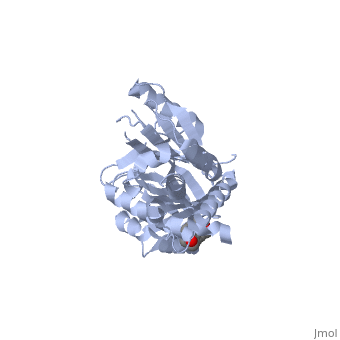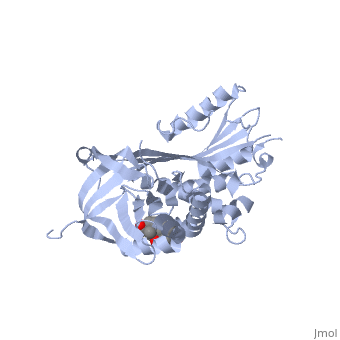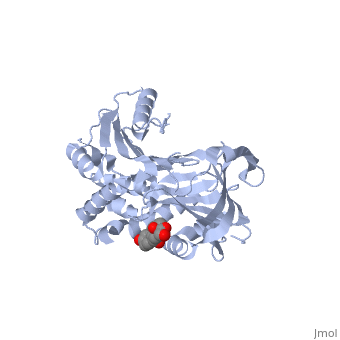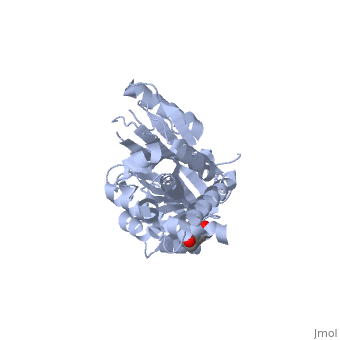
The structures show that the ligand binds in a , where the main interaction between cortisol and CBG is the pi-pi stacking between a key tryptophan residue (371 in humans), and the steroid ring of cortisol. There is also significant polar interaction between some of the polar side chains of the binding pocket residues and oxygen atoms on cortisol.
Key: Grey residues are hydrophobic, magenta residues are polar, the ligand is coloured orange
Functions
Because of its role as a hormone carrier, corticosteroid-binding globulin has been implicated in a wide range of biological functions, ranging from glucose metabolism to stress-responses to sperm motility [17],[18],[19]. However, the molecular mechanism of these are, arguably, not as well-understood as its role in the anti-inflammatory response, where its mode of action can be elegantly explained in the conformational changes described above.
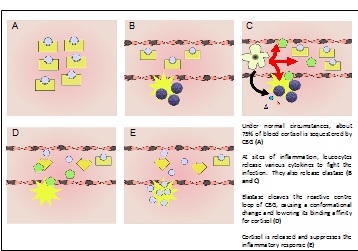
Proposed mechanism of cortisol release at sites of inflammation
Naturally-occurring genetic variants[20]
Leuven
CBG Leuven has an exon 2 point mutation which changes to a histidine, which reduces the binding affinity for cortisol by three-fold, although there appears to be no known clinical implications.
Lyon
CBG Lyon has an exon 5 point mutation which changes to an asparagine. This mutation lies close to the cortisol-binding pocket and reduces cortisol affinity by four-fold. Heterozygotes show reduction in immunoreactive CBG levels and total cortisol levels but were otherwise healthy, while homozygotes presented with fatigue, depressed mood and low blood pressure.
CBG Null
CBG Null is an exon 2 null mutation resulting in premature termination codon corresponding to residue -12 of pro-CBG molecule (G121A). This leads to 50% (heterozygotes) or undetectable (homozygotes) CBG levels, and both hetero- and homozygotes presented with chronic fatigue and low blood pressure
Conclusion
Corticosteroid-binding globulin is a very important blood protein that functions not merely as a passive carrier of cortisol, but also as a ready reservoir of the hormone for release at sites of inflammation. It also acts as a buffer to keep the level of blood cortisol constant and thus, maintains homeostasis. Work is still being done, not just in fully elucidating the molecular mechanism of its function, but also in its interaction with various tissues in the body, and this can have great implications on our understanding of metabolic diseases, as well as infection and immunity.
3D structures of corticosteroid-binding protein
Updated on 09-June-2015
2v95 – rSer-A6 – rat
2vdx – hSer-A6 (mutant) - human
2vdy – hSer-A6 (mutant) + cortisol
4bb2 – hSer-A6 (mutant) + progesterone
Resources
Nucleotide and amino acid sequence [1]
PDB structure of S-state rat CBG [2]
PDB structure of R-state human CBG-antitrypsin chimera [3]
PDB structure of R-state human CBG-antitrypsin chimera [4]
References
- ↑ WESTPHAL U. Steroid-protein interactions. III. Spectrophotometric demonstration of interaction between proteins and progesterone, deoxycorticosterone and cortisol. Arch Biochem Biophys. 1957 Jan;66(1):71-90. PMID:13395526
- ↑ Mickelson KE, Harding GB, Forsthoefel M, Westphal U. Steroid-protein interactions. Human corticosteroid-binding globulin: characterization of dimer and electrophoretic variants. Biochemistry. 1982 Feb 16;21(4):654-60. PMID:7074030
- ↑ Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988 Nov 17;336(6196):257-8. PMID:3143075 doi:http://dx.doi.org/10.1038/336257a0
- ↑ WESTPHAL U. Steroid-protein interactions. III. Spectrophotometric demonstration of interaction between proteins and progesterone, deoxycorticosterone and cortisol. Arch Biochem Biophys. 1957 Jan;66(1):71-90. PMID:13395526
- ↑ Mickelson KE, Harding GB, Forsthoefel M, Westphal U. Steroid-protein interactions. Human corticosteroid-binding globulin: characterization of dimer and electrophoretic variants. Biochemistry. 1982 Feb 16;21(4):654-60. PMID:7074030
- ↑ Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. J Biol Chem. 2007 Oct 5;282(40):29594-603. Epub 2007 Jul 19. PMID:17644521 doi:10.1074/jbc.M705014200
- ↑ Zhou A, Wei Z, Stanley PL, Read RJ, Stein PE, Carrell RW. The S-to-R transition of corticosteroid-binding globulin and the mechanism of hormone release. J Mol Biol. 2008 Jun 27;380(1):244-51. Epub 2008 May 14. PMID:18513745 doi:10.1016/j.jmb.2008.05.012
- ↑ Windle RJ, Wood SA, Lightman SL, Ingram CD. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology. 1998 Oct;139(10):4044-52. PMID:9751481
- ↑ Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988 Nov 17;336(6196):257-8. PMID:3143075 doi:http://dx.doi.org/10.1038/336257a0
- ↑ Torpy DJ, Ho JT. Corticosteroid-binding globulin gene polymorphisms: clinical implications and links to idiopathic chronic fatigue disorders. Clin Endocrinol (Oxf). 2007 Aug;67(2):161-7. Epub 2007 Jun 4. PMID:17547679 doi:10.1111/j.1365-2265.2007.02890.x
- ↑ Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. 2010 Mar 5;316(1):3-12. Epub 2009 Jul 28. PMID:19643161 doi:10.1016/j.mce.2009.06.015
- ↑ Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. J Biol Chem. 2007 Oct 5;282(40):29594-603. Epub 2007 Jul 19. PMID:17644521 doi:10.1074/jbc.M705014200
- ↑ Zhou A, Wei Z, Stanley PL, Read RJ, Stein PE, Carrell RW. The S-to-R transition of corticosteroid-binding globulin and the mechanism of hormone release. J Mol Biol. 2008 Jun 27;380(1):244-51. Epub 2008 May 14. PMID:18513745 doi:10.1016/j.jmb.2008.05.012
- ↑ Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. J Biol Chem. 2007 Oct 5;282(40):29594-603. Epub 2007 Jul 19. PMID:17644521 doi:10.1074/jbc.M705014200
- ↑ Zhou A, Wei Z, Stanley PL, Read RJ, Stein PE, Carrell RW. The S-to-R transition of corticosteroid-binding globulin and the mechanism of hormone release. J Mol Biol. 2008 Jun 27;380(1):244-51. Epub 2008 May 14. PMID:18513745 doi:10.1016/j.jmb.2008.05.012
- ↑ Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988 Nov 17;336(6196):257-8. PMID:3143075 doi:http://dx.doi.org/10.1038/336257a0
- ↑ Fernandez-Real JM, Grasa M, Casamitjana R, Pugeat M, Barret C, Ricart W. Plasma total and glycosylated corticosteroid-binding globulin levels are associated with insulin secretion. J Clin Endocrinol Metab. 1999 Sep;84(9):3192-6. PMID:10487686
- ↑ Teves ME, Guidobaldi HA, Unates DR, Sanchez R, Miska W, Giojalas LC. Progesterone sperm chemoattraction may be modulated by its corticosteroid-binding globulin carrier protein. Fertil Steril. 2010 May 1;93(7):2450-2. Epub 2009 Nov 6. PMID:19896663 doi:10.1016/j.fertnstert.2009.09.012
- ↑ Torpy DJ, Ho JT. Corticosteroid-binding globulin gene polymorphisms: clinical implications and links to idiopathic chronic fatigue disorders. Clin Endocrinol (Oxf). 2007 Aug;67(2):161-7. Epub 2007 Jun 4. PMID:17547679 doi:10.1111/j.1365-2265.2007.02890.x
- ↑ Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. 2010 Mar 5;316(1):3-12. Epub 2009 Jul 28. PMID:19643161 doi:10.1016/j.mce.2009.06.015