Journal:JBIC:18
From Proteopedia
(Difference between revisions)

| Line 11: | Line 11: | ||
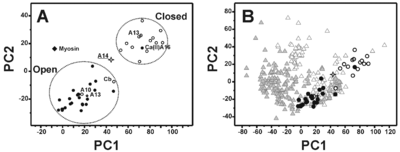
The most remarkable feature of this structural conformation involves the packing of the helices that is reduced with respect to the ‘closed’ structures of the S100 proteins but is still sizably larger than the corresponding ‘open’ structures. At the same time, the analysis of the electrostatic potential surface suggests that the <scene name='Journal:JBIC:18/Cv/2'>S100A14</scene> is <scene name='Journal:JBIC:18/Cv/7'>permanently activated and it is not calcium(II) regulated</scene>. | The most remarkable feature of this structural conformation involves the packing of the helices that is reduced with respect to the ‘closed’ structures of the S100 proteins but is still sizably larger than the corresponding ‘open’ structures. At the same time, the analysis of the electrostatic potential surface suggests that the <scene name='Journal:JBIC:18/Cv/2'>S100A14</scene> is <scene name='Journal:JBIC:18/Cv/7'>permanently activated and it is not calcium(II) regulated</scene>. | ||
| + | PDB reference: [[2m0r]]. | ||
</StructureSection> | </StructureSection> | ||
Current revision
| |||||||||||
- ↑ Bertini I, Borsi V, Cerofolini L, Das Gupta S, Fragai M, Luchinat C. Solution structure and dynamics of human S100A14. J Biol Inorg Chem. 2012 Nov 30. PMID:23197251 doi:10.1007/s00775-012-0963-3
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.