This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Michael Roberts/BIOL115 CaM
From Proteopedia
| Line 3: | Line 3: | ||
'''Sequence and structure of EF hands''' | '''Sequence and structure of EF hands''' | ||
| + | <Structure load='1CLL' size='500' frame='true' align='right' caption='Human calmodulin' scene='Insert optional scene name here' /> | ||
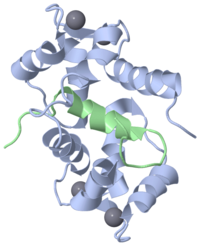
The EF hand motif is present in a many proteins and it commonly bestows the ability to bind Ca2+ ions. It was first identified in parvalbumin, a muscle protein. Here we will have a look at the Ca2+-binding protein calmodulin, which possesses four EF hands. Calmodulin and its isoform, troponinC, are important intracellular Ca2+-binding proteins. | The EF hand motif is present in a many proteins and it commonly bestows the ability to bind Ca2+ ions. It was first identified in parvalbumin, a muscle protein. Here we will have a look at the Ca2+-binding protein calmodulin, which possesses four EF hands. Calmodulin and its isoform, troponinC, are important intracellular Ca2+-binding proteins. | ||
The structure on the right, obtained by X-ray crystallography, represents the Ca2+-binding protein calmodulin. It has a dumbell-shaped structure with two identical lobes connected by a central alpha-helix. Each lobe comprises three a helices joined by loops. A helix-loop-helix motif forms the basis of each EF hand. | The structure on the right, obtained by X-ray crystallography, represents the Ca2+-binding protein calmodulin. It has a dumbell-shaped structure with two identical lobes connected by a central alpha-helix. Each lobe comprises three a helices joined by loops. A helix-loop-helix motif forms the basis of each EF hand. | ||
Click on the 'green links' below to examine this molecule in more detail. | Click on the 'green links' below to examine this molecule in more detail. | ||
| - | |||
| - | <Structure load='1CLL' size='500' frame='true' align='right' caption='Human calmodulin' scene='Insert optional scene name here' /> | ||
Let us color the two main forms of regular <scene name='Sandbox_LUBIOL115/Structure_plus_ca/1'>secondary structure</scene> in this protein. Alpha helix appears in red, beta sheet in yellow. | Let us color the two main forms of regular <scene name='Sandbox_LUBIOL115/Structure_plus_ca/1'>secondary structure</scene> in this protein. Alpha helix appears in red, beta sheet in yellow. | ||
Revision as of 23:30, 11 April 2013
Sequence and structure of EF hands
|
The EF hand motif is present in a many proteins and it commonly bestows the ability to bind Ca2+ ions. It was first identified in parvalbumin, a muscle protein. Here we will have a look at the Ca2+-binding protein calmodulin, which possesses four EF hands. Calmodulin and its isoform, troponinC, are important intracellular Ca2+-binding proteins. The structure on the right, obtained by X-ray crystallography, represents the Ca2+-binding protein calmodulin. It has a dumbell-shaped structure with two identical lobes connected by a central alpha-helix. Each lobe comprises three a helices joined by loops. A helix-loop-helix motif forms the basis of each EF hand.
Click on the 'green links' below to examine this molecule in more detail.
Let us color the two main forms of regular in this protein. Alpha helix appears in red, beta sheet in yellow.
Alpha Helices, Beta Strands , Turns.