We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Non-polymerizable monomeric actin
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
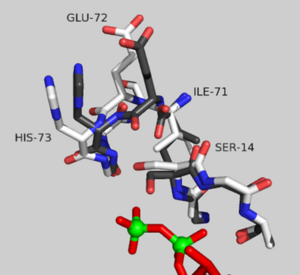
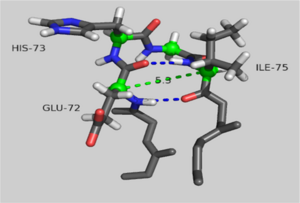
Structural changes between the ATP and ADP-bound state of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_all_activesite/1'> AP-actin </scene> are confined to the active site. The <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_scene2/3'> alpha-carbon backbones </scene> of the two states outside the sensor loop region superimpose very well, with a RMSD of 0.19 angstroms. A close-up of the <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop/5'> active site </scene> reviles how binding ATP elicits structural changes in the nucleotide-binding cleft that is propagated to the sensor loop. When actin is bound to ADP, the serine-14 side chain forms a hydrogen bind with the beta phosphate ADP. Upon ATP binding, the gamma phosphate sterically clashes with the oxygen of serine-14, forcing the hydroxyl to rotate 130°. The displaced serine residue impinges on the backbone carbonyls of residues isoleucine-71 and glutamic acid-72 in the sensor loop. This causes a 180° rotation of the peptide linkage between glutamic acid-72 and histidine-73. These structural changes impact residues proximal to the sensor loop. Transition to the ATP-bound state causes glutamic acid-72 to form a new hydrogen bond with threonine-77. This induces a reorientation of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/5'>asparagine-78</scene>. ATP binding also disrupts hydrogen bonding between arginine-183 and residues 72 and 73 of the sensor loop causing a conformational change of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/6'>arginine-183</scene>. Aspartic acid-179 stacks against histidine-73 in the ATP-bound state which induces a shift in the position of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/7'>arginine-177 </scene> to improve salt bridge formation with aspartic acid-179. In the AP-actin structure, changes in conformation due ATP binding are local to the active site and do not appear to propagate into domains. | Structural changes between the ATP and ADP-bound state of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_all_activesite/1'> AP-actin </scene> are confined to the active site. The <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_scene2/3'> alpha-carbon backbones </scene> of the two states outside the sensor loop region superimpose very well, with a RMSD of 0.19 angstroms. A close-up of the <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop/5'> active site </scene> reviles how binding ATP elicits structural changes in the nucleotide-binding cleft that is propagated to the sensor loop. When actin is bound to ADP, the serine-14 side chain forms a hydrogen bind with the beta phosphate ADP. Upon ATP binding, the gamma phosphate sterically clashes with the oxygen of serine-14, forcing the hydroxyl to rotate 130°. The displaced serine residue impinges on the backbone carbonyls of residues isoleucine-71 and glutamic acid-72 in the sensor loop. This causes a 180° rotation of the peptide linkage between glutamic acid-72 and histidine-73. These structural changes impact residues proximal to the sensor loop. Transition to the ATP-bound state causes glutamic acid-72 to form a new hydrogen bond with threonine-77. This induces a reorientation of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/5'>asparagine-78</scene>. ATP binding also disrupts hydrogen bonding between arginine-183 and residues 72 and 73 of the sensor loop causing a conformational change of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/6'>arginine-183</scene>. Aspartic acid-179 stacks against histidine-73 in the ATP-bound state which induces a shift in the position of <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_morph_sensorloop_add/7'>arginine-177 </scene> to improve salt bridge formation with aspartic acid-179. In the AP-actin structure, changes in conformation due ATP binding are local to the active site and do not appear to propagate into domains. | ||
| - | |||
| - | <table width='300' align='right' cellpadding='5'><tr><td rowspan='2'> </td><td bgcolor='#d0d0d0'><applet load=Image:10state morph of 1ATN 1J6Z.pdb' size='300' align='right' scene='User:Thomas_E_Sladewski/Sandbox_1/10state_d_loop_morph/2' /></td></tr><tr><td bgcolor='#d0d0d0'>Morph of subdomain 2 of actin complexed with TMR in the ADP state and actin complexed with DNAse I in the ATP state<ref name="TMR"/>.</td></tr></table> | ||
| - | |||
[[Image:2hf4 d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-bound AP-actin, (grey ribbon) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | [[Image:2hf4 d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-bound AP-actin, (grey ribbon) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | ||
[[Image:1J6Z d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-actin, complexed with TMR (grey ribbon)(PDB entry [[1J6Z]]) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | [[Image:1J6Z d loop crystal packing.png|250px|left|thumb| Crystal packing interactions between ADP-actin, complexed with TMR (grey ribbon)(PDB entry [[1J6Z]]) and neighboring residues in subdomain 2 of an adjacent monomer in the crystal within 15 angstroms of the D-loop in the unit cell (blue ribbons). Residues 39-50 are shown in red.]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
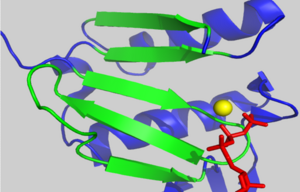
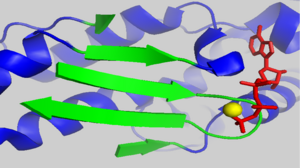
===The D-loop=== | ===The D-loop=== | ||
| - | + | <scene name='User:Thomas_E_Sladewski/Sandbox_1/10state_d_loop_morph/2'>Morph of subdomain 2 of actin complexed with TMR in the ADP state and actin complexed with DNAse I in the ATP state</scene> <ref name="TMR"/>. | |
There is some controversy over whether or not the D-loop undergoes structural changes upon actin binding ATP. In the structure of AP-actin, the D-loop is disordered in both the ATP and ADP-bound state. Also, there is no evidence that structural changes in the nucleotide binding cleft propagate to subdomain 2. This argues that the D-loop remains disordered in both states. However, other groups show large ATP-dependent structural changes in the D-loop<ref name="TMR">PMID:11474115</ref>. This is illustrated, right, in a subdomain 2 morph of actin complexed with tetramethylrhodamine (TMR) in the ADP-bound state, (PDB entry [[1J6Z]]) and actin complexed with DNAase I in the ATP-bound state, (PDB entry [[1ATN]])<ref name="TMR"/>. These structures revile that the D-loop is disordered when actin is bound to ATP, and transitions to an alpha-helix in the ADP-bound state. It has been suggested that the alpha helix in the ADP-bound state results from crystal packing. In support of this, actin complexed with TMR in the ADP state shows extensive neighboring contacts around the D-loop (shown left, lower panel). In contrast, AP-actin, (PDB entry [[2HF3]]), shows far fewer crystal contacts around the D-loop (shown left, upper panel). These crystal contacts have been proposed to result in the nucleotide-dependent structural changes in the D-loop observed in some structures. In further support of this, the sequence of the D-loop, HQGVMVGMG, has a low propensity to form an alpha helix. However, molecular dynamic simulations show that the D-loop favors the alpha helix conformation in the ADP state, and not the ATP or ADP-Pi states <ref name="zheng">PMID:17526584</ref>. This study supports a model where small perturbations in the active site shift the equilibrium of the D-loop between the flexible coil and helix state. Further studies are needed to resolve this question. | There is some controversy over whether or not the D-loop undergoes structural changes upon actin binding ATP. In the structure of AP-actin, the D-loop is disordered in both the ATP and ADP-bound state. Also, there is no evidence that structural changes in the nucleotide binding cleft propagate to subdomain 2. This argues that the D-loop remains disordered in both states. However, other groups show large ATP-dependent structural changes in the D-loop<ref name="TMR">PMID:11474115</ref>. This is illustrated, right, in a subdomain 2 morph of actin complexed with tetramethylrhodamine (TMR) in the ADP-bound state, (PDB entry [[1J6Z]]) and actin complexed with DNAase I in the ATP-bound state, (PDB entry [[1ATN]])<ref name="TMR"/>. These structures revile that the D-loop is disordered when actin is bound to ATP, and transitions to an alpha-helix in the ADP-bound state. It has been suggested that the alpha helix in the ADP-bound state results from crystal packing. In support of this, actin complexed with TMR in the ADP state shows extensive neighboring contacts around the D-loop (shown left, lower panel). In contrast, AP-actin, (PDB entry [[2HF3]]), shows far fewer crystal contacts around the D-loop (shown left, upper panel). These crystal contacts have been proposed to result in the nucleotide-dependent structural changes in the D-loop observed in some structures. In further support of this, the sequence of the D-loop, HQGVMVGMG, has a low propensity to form an alpha helix. However, molecular dynamic simulations show that the D-loop favors the alpha helix conformation in the ADP state, and not the ATP or ADP-Pi states <ref name="zheng">PMID:17526584</ref>. This study supports a model where small perturbations in the active site shift the equilibrium of the D-loop between the flexible coil and helix state. Further studies are needed to resolve this question. | ||
| Line 79: | Line 71: | ||
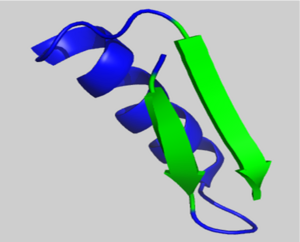
Subomain 1 contains a right handed β-α-β motif (residues 103-136). Shown left, two parallel β-strands are linked by an α-helix. These β-strands are part of the β-sheet motif in subdomain 1. | Subomain 1 contains a right handed β-α-β motif (residues 103-136). Shown left, two parallel β-strands are linked by an α-helix. These β-strands are part of the β-sheet motif in subdomain 1. | ||
| - | + | </StructureSection> | |
==See Also== | ==See Also== | ||
*[[Actin]] | *[[Actin]] | ||
Revision as of 07:56, 8 May 2013
| |||||||||||
See Also
References
- ↑ Rould MA, Wan Q, Joel PB, Lowey S, Trybus KM. Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J Biol Chem. 2006 Oct 20;281(42):31909-19. Epub 2006 Aug 18. PMID:16920713 doi:M601973200
- ↑ 2.0 2.1 2.2 2.3 Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science. 2001 Jul 27;293(5530):708-11. PMID:11474115 doi:10.1126/science.1059700
- ↑ Zheng X, Diraviyam K, Sept D. Nucleotide effects on the structure and dynamics of actin. Biophys J. 2007 Aug 15;93(4):1277-83. Epub 2007 May 25. PMID:17526584 doi:10.1529/biophysj.107.109215
- ↑ Buchberger A, Schroder H, Buttner M, Valencia A, Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994 Feb;1(2):95-101. PMID:7656024
- ↑ Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623-8. PMID:2143562 doi:http://dx.doi.org/10.1038/346623a0
- ↑ Kabsch W, Holmes KC. The actin fold. FASEB J. 1995 Feb;9(2):167-74. PMID:7781919
- ↑ van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001 Sep 6;413(6851):39-44. PMID:11544518 doi:http://dx.doi.org/10.1038/35092500
- ↑ Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993 Sep 5;233(1):123-38. PMID:8377180 doi:http://dx.doi.org/10.1006/jmbi.1993.1489
- ↑ Aurora R, Rose GD. Helix capping. Protein Sci. 1998 Jan;7(1):21-38. PMID:9514257 doi:10.1002/pro.5560070103