Yersinia YopH
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='1xxv' size=' | + | <StructureSection load='1xxv' size='350' side='right' scene='' caption='YopH (grey, green) complex with EGFR peptide (pink, yellow, magenta, cyan) (PDB code [[1xxv]])'> |
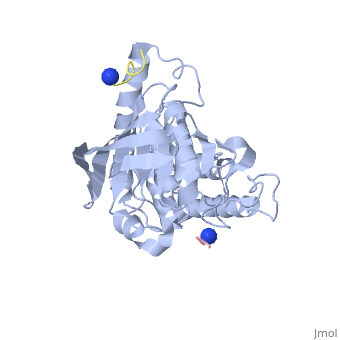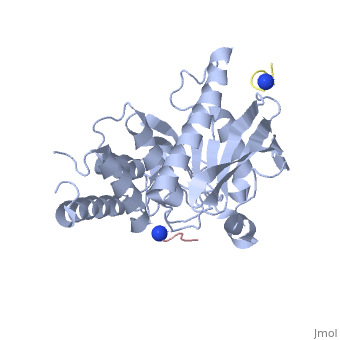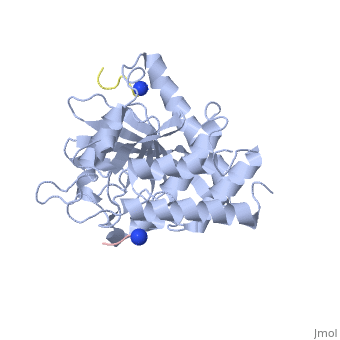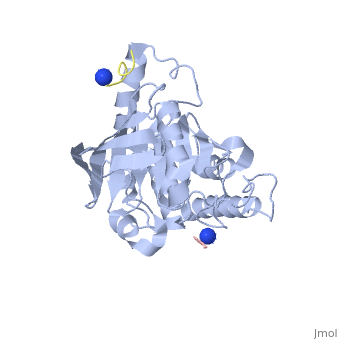
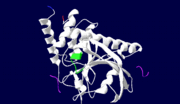
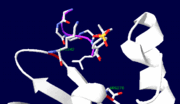
[[Yersinia YopH]] (Yop51) is a virulence factor of bacteria of the genus ''Yersinia'', which includes ''Y. pestis'' (which can manifest in one form as bubonic plague), ''Y. pseudotuberculosis'', and ''Y. enterocolitica''. YopH is a protein tyrosine phosphatase (PTPase) which is injected into infected cells by a type III secretion mechanism<ref name="Ivanov">Ivanov, M.I., Stuckey, J.A., Schubert, H.L. Saper, M.A., Bliska, J.B. Two substrate-targeting sites in the ''Yersinia'' protein tyrosine phosphatase co-operate to promote bacterial virulence. ''Molecular Microbiology''. 2005. 55:1346-1356.</ref><ref name="Persson"/><ref name="Black"> Black, D.S., and Bliska, J.B. Identification of p130Cas as a substrate of ''Yersinia'' YopH(Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. ''The EMBO Journal''. 1997. 16:2730-2744.</ref> <scene name='39/399846/Cv/1'>Crystal structure showing two YopH protein-tyrosine phosphatase catalytic domains from Yersinia enterocolitica bound to EGFR-derived peptides</scene> ([[1xxv]]). | [[Yersinia YopH]] (Yop51) is a virulence factor of bacteria of the genus ''Yersinia'', which includes ''Y. pestis'' (which can manifest in one form as bubonic plague), ''Y. pseudotuberculosis'', and ''Y. enterocolitica''. YopH is a protein tyrosine phosphatase (PTPase) which is injected into infected cells by a type III secretion mechanism<ref name="Ivanov">Ivanov, M.I., Stuckey, J.A., Schubert, H.L. Saper, M.A., Bliska, J.B. Two substrate-targeting sites in the ''Yersinia'' protein tyrosine phosphatase co-operate to promote bacterial virulence. ''Molecular Microbiology''. 2005. 55:1346-1356.</ref><ref name="Persson"/><ref name="Black"> Black, D.S., and Bliska, J.B. Identification of p130Cas as a substrate of ''Yersinia'' YopH(Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. ''The EMBO Journal''. 1997. 16:2730-2744.</ref> <scene name='39/399846/Cv/1'>Crystal structure showing two YopH protein-tyrosine phosphatase catalytic domains from Yersinia enterocolitica bound to EGFR-derived peptides</scene> ([[1xxv]]). | ||
Revision as of 07:51, 29 February 2016
| |||||||||||
3D structures of protein tyrosine phosphatase
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Ivanov, M.I., Stuckey, J.A., Schubert, H.L. Saper, M.A., Bliska, J.B. Two substrate-targeting sites in the Yersinia protein tyrosine phosphatase co-operate to promote bacterial virulence. Molecular Microbiology. 2005. 55:1346-1356.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Persson, C. Carballeira, N., Wolf-Watz, H., and Fällman, M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of pl30cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. The EMBO Journal. 1997. 16:2307-2318.
- ↑ 3.0 3.1 Black, D.S., and Bliska, J.B. Identification of p130Cas as a substrate of Yersinia YopH(Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. The EMBO Journal. 1997. 16:2730-2744.
- ↑ 4.0 4.1 4.2 4.3 4.4 Hamid, N., Gustavsson, A.,Andersson, K., McGee, K., Persson, C., Rudd, C.E., Fällman, M. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microbial Pathogenesis. 1999. 26:231-242
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Galán, J.E. and Collmer, A. Type III Secretion Machines: Bacterial Devices for Protein Delivery into Host Cells. Science. 1999. 284:1322-1328
- ↑ 6.0 6.1 Wulff-Strobel, C.R., Williams, A.W., Straley, S.C. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Molecular Microbiology.2002. 43:411-423
- ↑ 7.0 7.1 7.2 Cornelis, G.R. and Wolf-Watz, H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Molecular Microbiology. 1997. 23:861-867.
- ↑ 8.0 8.1 8.2 8.3 . Stuckey, J.A., Schubert, H.L., Fauman, E.B., Zhang, Z., Dixon, J.E., and Saper, M.A. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5Å and the complex with tungstate. Nature. 1994. 370:571-575.
- ↑ 9.0 9.1 9.2 9.3 Smith, C.L., Khandelwal, P., Keliikuli, K., Zuiderweg, E.R.P. and Saper, M. Structure of the type III secretion and substrate-binding domain of Yersinia YopH phosphatase. Molecular Microbiology. 2001. 42:967-979.
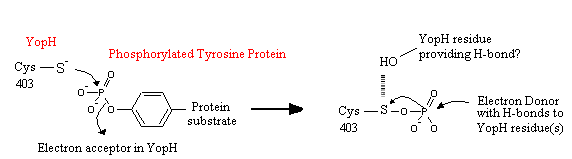
- ↑ 10.0 10.1 Pannifer, A.D.B., Flint, F.J., Tonks, N.K., and Barford, D. Visualization of the Cysteinyl-phosphate Intermediate of a Protein-tyrosine Phosphatase by X-ray Crystallography. The Journal of Biological Chemistry. 1998. 273:10454-10462.
- ↑ 11.0 11.1 DeVinney, R., Steele-Mortimer, O., and Finlay, B.B. Phosphatases and kinases delivered to the host cell by bacterial pathogens. Trends in Microbiology. 2000. 8:29-33.
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Cara Halseth, Alexander Berchansky