We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 126
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Background == | == Background == | ||
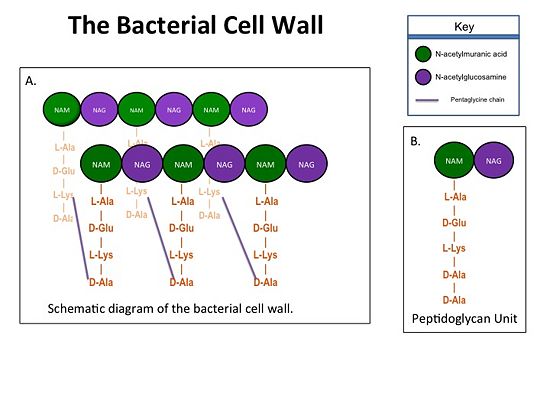
| - | Transpeptidases (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. The natural transpeptidase substrate is the D-Ala-D-Ala peptidoglycan side chain terminus. Beta-lactam (β-lactam) antibiotics, which include penicillins, cephalosporins and carbapenems, bind and irreversibly inhibit transpeptidases by mimicking the D-Ala-D-Ala substrate, resulting in the inhibition of cell wall synthesis and ultimately bacterial cell growth. [[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate | | + | Transpeptidases (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. The natural transpeptidase substrate is the D-Ala-D-Ala peptidoglycan side chain terminus. Beta-lactam (β-lactam) antibiotics, which include penicillins, cephalosporins and carbapenems, bind and irreversibly inhibit transpeptidases by mimicking the D-Ala-D-Ala substrate, resulting in the inhibition of cell wall synthesis and ultimately bacterial cell growth. [[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate |550px]] Overuse and misuse of β-lactams has led to the generation of methicillin- resistant Staphylococcus aureus (MRSA) isolates that have acquired an alternative transpeptidase, PBP2a, which is neither bound nor inhibited by β- lactams. MRSA isolates are resistant to all β-lactams, and are often only susceptible to “last resort antibiotics”, such as vancomycin. Recently, two cephalosporins - <scene name='37/372726/Ceftobiprole/2'>Ceftobiprole</scene> and ceftaroline - that bind and inhibit PBP2a have been developed. |
<scene name='37/372726/Pbp2a_with_residues/1'>PBP2a and active site residues</scene> | <scene name='37/372726/Pbp2a_with_residues/1'>PBP2a and active site residues</scene> | ||
| - | <scene name='37/372726/Close_up_of_active_site/4'> | + | <scene name='37/372726/Close_up_of_active_site/4'>active site</scene> |
| - | <scene name='37/372726/ | + | ==How does PBP2a work== |
| + | PBP2a is composed of two domains: a <scene name='37/372726/Tp_and_npb_domain/1'>non-penicillin binding (NPB) domain and a TP domain</scene> The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain “sits” in the periplasm with its active site facing the inner surface of the cell wall. The active site contains a serine residue at position 403 (Ser403) which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. | ||
| - | <scene name='37/372726/Ceftobiprole/4'>Ceftobiproles R2 Group</scene> | ||
| + | == How does Ceftobiprole Work == | ||
| - | + | <scene name='37/372726/Ceftobiprole/2'>Ceftobiprole</scene> is able to inhibit PBP2a because additional chemical groups at <scene name='37/372726/Ceftobiprole/4'>the R2 position of the cephalosporin backbone</scene> are able to interact with additional amino acid residues in PBP2a; specifically Tyr446 and Met641. As a result of its tighter binding to PBP2a, ceftobiprole is able to more efficiently react with the serine active site residue and therefore inhibit the activity of PBP2a. | |
| - | = | + | |
| - | + | ||
Revision as of 18:41, 30 July 2013
| |||||||||||