Matrix metalloproteinase
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image: | + | <StructureSection load='M1.pdb' size='500' side='right' scene='MT1-MMP-TIMP-1_complex/Cv2/8' caption=''> |
| - | {{ | + | [[Image:3ma2a.png|left|300px|thumb|MT1-MMP-TIMP-1 complex]] |
| - | + | {{Clear}} | |
'''Matrix metalloproteinases''' (MMP) are Zinc-dependent endopeptidases. MMP degrades extracellular matrix proteins. MMPs are produced by 28 different genes and are classified according to their protein substrates. They are inhibited by proteases called tissue inhibitors of metalloproteinase (TIMP). The pro-MMP contains a pro-peptide which must be removed to render the MMP active. See details of MMP12 in [[Matrix Metalloproteinase 12]]. More details of MMP in<br /> | '''Matrix metalloproteinases''' (MMP) are Zinc-dependent endopeptidases. MMP degrades extracellular matrix proteins. MMPs are produced by 28 different genes and are classified according to their protein substrates. They are inhibited by proteases called tissue inhibitors of metalloproteinase (TIMP). The pro-MMP contains a pro-peptide which must be removed to render the MMP active. See details of MMP12 in [[Matrix Metalloproteinase 12]]. More details of MMP in<br /> | ||
[[Matrix metalloproteinases]]<br /> | [[Matrix metalloproteinases]]<br /> | ||
Revision as of 13:28, 14 August 2013
| |||||||||||
3D structures of matrix metalloproteinase
Updated June 2012
MMP1 interstitial or fibroblast collagenase
1su3 – pro-hMMP – human
2clt – hMMP (mutant)
3shi, 1hfc - hMMP catalytic domain
2tcl, 966c - hMMP catalytic domain + inhibitor
2ayk, 3ayk, 4ayk - hMMP catalytic domain - NMR
1fbl – MMP - pig
MMP2 gelatinase-A
1qib, 1ck7 - hMMP catalytic domain (mutant)
1rtg - hMMP hemopexin-like domain
1ks0 – hMMP first fibronectin type II domain – NMR
1cxw - hMMP second fibronectin type II domain – NMR
1j7m - hMMP third fibronectin type II domain (mutant) – NMR
1gen – hMMP C terminal
1eak – pro-hMMP catalytic domain (mutant) + peptide inhibitor
3ayu - hMMP catalytic domain (mutant) + peptide inhibitor
1hov, 1eub - hMMP catalytic domain + inhibitor– NMR
1gxd – pro-hMMP (mutant) + TIMP-2
MMP3 stromelysin 1
1qia, 1qic, 1cqr, 1slm - hMMP catalytic domain
3ohl, 3oho, 1g49, 1ciz, 1b8y, 1caq, 1usn, 2usn, 1ums, 1umt, 2d1n, 2d1o, 2ow9, 1bqo, 1g4k, 1b3d, 1biw, 1c3i, 1d5j, 1d7x, 1d8f, 1d8m, 1g05, 1hfs, 1hy7, 2srt- hMMP catalytic domain + inhibitor
1c8t - hMMP catalytic domain (mutant) + inhibitor
1uea - hMMP catalytic domain + TIMP-1
1oo9 - hMMP catalytic domain + TIMP-1 N terminal
2jt5, 2jt6, 2jnp, 3usn, 1sln, 1bm6 - hMMP catalytic domain + inhibitor – NMR
MMP7 matrilysin
2y6c, 2y6d, 2ddy, 1mmp, 1mmq, 1mmr – hMMP catalytic domain + inhibitor
MMP8 neutrophil collagenase
2oy4, 1mnc - hMMP catalytic domain
3dng, 3dpe, 3dpf, 1zp5, 1jh1, 1jj9, 1i76, 1a85, 1mmb, 1zs0, 1zvx, 1lbc – hMMP catalytic domain + inhibitor
1i73, 2oy2, 1jan, 1jao, 1jap, 1jaq - hMMP catalytic domain + peptide inhibitor
1a86 - hMMP catalytic domain + aspartate-based inhibitor
MMP9 gelatinase-B
1l6j - pro-hMMP
1itv – hMMP haemopexin-like domain
1gkc - hMMP catalytic domain + inhibitor
2ovx, 2ovz, 2ow0, 2ow1, 2ow2, 1gkd - hMMP catalytic domain (mutant) + inhibitor
MMP10 stromelysin 2
1q3a - hMMP catalytic domain (mutant)
3v96 - hMMP catalytic domain + metalloproteinase inhibitor
MMP11 stromelysin 3
1hv5 - hMMP catalytic domain + inhibitor
MMP12 macrophage
3ba0, 2oxu - hMMP
2krj, 2k9c - hMMP catalytic domain – NMR
1jk3, 1jiz, 2oxu - hMMP catalytic domain
2poj - hMMP catalytic domain (mutant) - NMR
2jxy - hMMP hemopexin-like domain - NMR
3n2u, 3n2v, 2wo8, 2wo9, 2woa, 1utt, 1utz, 1ros – hMMP catalytic domain + inhibitor
3lk8, 3lik, 3lil, 3lir, 3ljg, 3nx7, 3lka, 3ehx, 3ehy, 3f15, 3f16, 3f17, 3f18, 3f19, 3f1a, 1y93, 1rmz, 1os2, 1os9, 2hu6 - hMMP catalytic domain (mutant) + inhibitor
2oxn, 2oxz, 2oxw - hMMP catalytic domain (mutant) + peptide
2k2g, 2z2d - hMMP catalytic domain + inhibitor - NMR
2w0d, 1ycm, 1z3j - hMMP catalytic domain (mutant) + inhibitor - NMR
MMP13 collagenase 3
1cxv - MMP catalytic domain - mouse
1pex – hMMP hemopexin-like domain
2yig, 3ljz, 3kec, 3kej, 3kek, 3kry, 3i7g, 3i7i, 3elm, 2pjt, 2ozr, 1xuc, 1xud, 1xur, 1you, 1ztq, 3o2x, 3zxh, 4a7b, 1fls, 1fm1, 456c, 830c – hMMP catalytic domain + inhibitor
2e2d - hMMP catalytic domain + TIMP-2
MMP14 Membrane T1
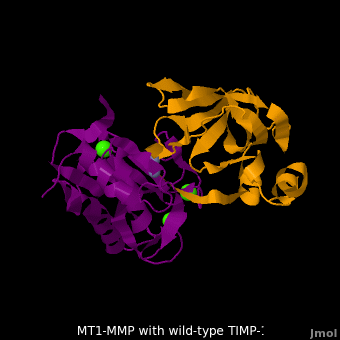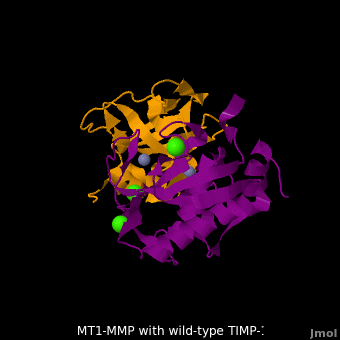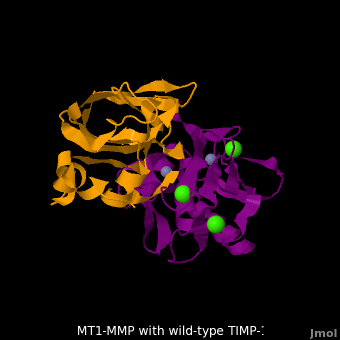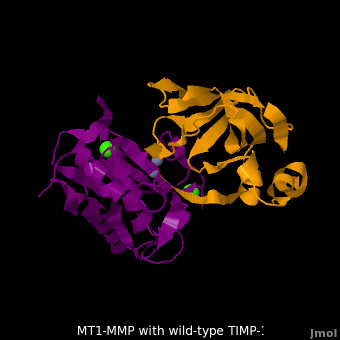
3ma2 – hMMP residues 112-292 + TIMP-1 (mutant)
1buv, 1bqq - hMMP + TIMP-2
3c7x – hMMP hemopexin-like domain
MMP16 Membrane T3
1rm8 - hMMP catalytic domain + inhibitor
MMP20 enamelysin
2jsd - hMMP catalytic domain + inhibitor - NMR
MMP23 CA-MMP
2k72 – hMMP residues 254-290 - NMR
MMP adamalysin
1iag – MMP – diamondback rattlesnake
References
- ↑ Grossman M, Tworowski D, Dym O, Lee MH, Levy Y, Murphy G, Sagi I. Intrinsic protein flexibility of endogenous protease inhibitor TIMP-1 controls its binding interface and effects its function. Biochemistry. 2010 Jun 14. PMID:20545310 doi:10.1021/bi902141x