We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 22222
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load= size=475 side='right' scene=' | + | <StructureSection load= size=475 side='right' scene='36/365380/4dki_cartoon/19'> |
| - | + | ||
| - | + | ||
| + | Transpeptidase (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. Beta-lactam (β-lactam) antibiotics, which | ||
| + | include penicillins, cephalosporins and carbapenems, bind and irreversibly inhibit transpeptidases. The overuse and misuse of β-lactam antibiotics has led to strains of Staphylococcus aureus that are resistant to all β-lactams and are often only susceptible to “last resort antibiotics”, such as vancomycin. | ||
| - | ==='''Identification of the cargo and Transport through the NPC'''=== | ||
| - | In eukaryotes, proteins must be transported in and out of the nucleus. This nucleocytoplasmic transport of proteins across the nuclear envelope must occur through the gateway of the NPC. The NPC is a large structure consisting of 456 constituent binding proteins called nucleoporins (Nups).1 Movement through the NPC is facilitated transport that relies on interaction with specific Nups. Importins and exportins are proteins that aid this facilitated transport by both binding to a specific cargo to be transported and interacting with specific Nups located in the central channel of the NPC.2 | ||
| - | + | == Cell Wall Structure == | |
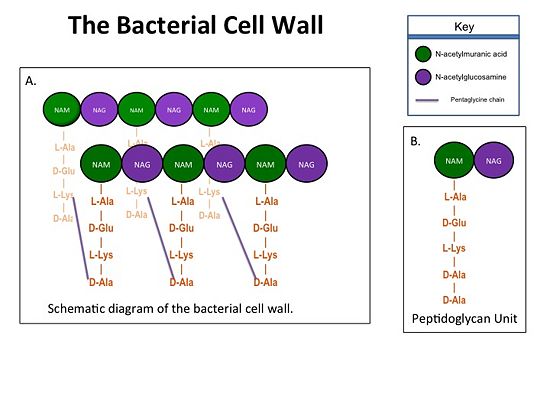
| - | + | The cell wall, which is composed of peptidoglycan, is crucial for maintaining the structural integrity of the bacterium. Peptidoglycan consists of N-acetylmuramic Acid (NAM) and N-acetylglucosamine (NAG) polymers. Rows of peptidoglycan are cross-linked together with pentaglycine chains. The NAM residues have a five amino acid side chain that terminates with two D-Alanine (D-Ala) residues. | |
| + | [[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate |550px]] | ||
| - | + | == Structure of a Resistant Transpeptidase == | |
| - | <scene name=' | + | Methicillin resistant Staphylococcus aureus (MRSA) is resistant to all β-lactams because it acquires an alternative PBP, PBP2a, that is not bound or inhibited by any β-lactams. PBP2a is composed of two domains: a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/20'>(NPB) </scene></font> and a <font color='dodgerblue'><b>transpeptidase <scene name='36/365380/4dki_cartoon/21'>(TP)</scene> binding domain </b></font>. The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain “sits” in the periplasm with its active site facing the inner surface of the cell wall. The active site contains <scene name='36/365380/Ser403/19'>a serine residue at position 403 (ser403)</scene> which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. |
| - | <!-- | ||
| - | --> | ||
| - | [[ | + | == Catalytic Mechanism of PBP2a == |
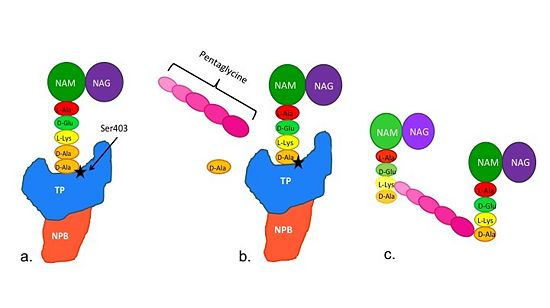
| + | [[Image:Schematic TP 3steps.jpg|thumb|alt= Alt text|Figure 2. Schematic diagram illustrating the mechanism of action of PBP2a |550px]] | ||
| + | |||
| + | (a)The D-Ala-D-Ala side-chain substrate of the peptidoglycan accesses the active site of the PBP2a. | ||
| + | |||
| + | (b)Ser403 nucleophilically attacks the peptide bond of the terminal D-Ala residues of the substrate. The terminal D-Ala residue then exits the active site. The now terminal D-Ala residue forms a covalent bond to Ser403, while a crosslinking pentaglycine chain enters the active site. | ||
| + | |||
| + | (c)A covalent bond forms between the pentaglycine chain and the terminal D-Ala residue, regenerating the active site serine residue. | ||
| + | |||
| + | The entire process takes 4 milliseconds. | ||
| + | |||
| + | == How Do Antibiotics Work? == | ||
| + | |||
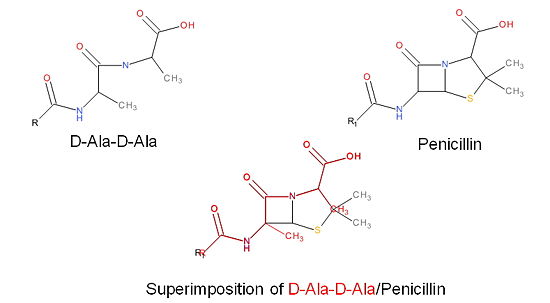
| + | The β-lactam antibiotics inhibit bacterial growth by inhibiting PBPs and ultimately cell wall | ||
| + | synthesis. Specifically, β-lactams are molecular mimics of D-Ala-D-Ala, which is the normal | ||
| + | substrate of PBPs. Nucleophillic attack of the β-lactam results in the PBP being irreversibly | ||
| + | inhibited by the β-lactam. As a result, the synthesis of the cell wall is inhibited which leads | ||
| + | to cell lysis. | ||
| + | |||
| + | [[Image:Structures on penicillin and b lactam.jpg|thumb|alt= Alt text|Figure 3. Mechanism of action of β-lactams. A. Structure of a β-lactam (penicillin) showing the amide, carboxyl, and β-lactam ring groups β-lactam ring groups. B. Structure of the D-Ala-D-Ala substrate. C. Overlay of the D-Ala-D-Ala substrate in red with penicillin demonstrating molecular mimicry.|550 px]] | ||
| + | |||
| + | |||
| + | |||
| + | == PBP2a and Ceftobiprole == | ||
| + | |||
| + | MRSA becomes resistant to β-lactams by acquiring an alternative PBP, PBP2a, that is | ||
| + | neither bound nor inhibited by β-lactams. Recently, two cephalosporins – <scene name='36/365380/Ceftobiprole/23'>ceftobiprole</scene> and | ||
| + | ceftaroline – that have anti-MRSA activity have been developed. Ceftobiprole is able to | ||
| + | inhibit PBP2a because additional chemical groups at the <scene name='36/365380/Ceftobiprole/12'>R2</scene> position of the cephalosporin backbone are able to interact with additional amino acid residues in PBP2a; specifically | ||
| + | <scene name='36/365380/Ceftobiprole/22'>Tyr446 and Met641</scene>. As a result of its tighter binding to PBP2a, ceftobiprole is able to more | ||
| + | efficiently react with the serine active site residue and therefore inhibit the activity of | ||
| + | PBP2a. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | MRSA becomes resistant to β-lactams by acquiring an alternative PBP, PBP2a, that is | ||
| + | neither bound nor inhibited by β-lactams. Recently, two cephalosporins – | ||
| + | <scene name='37/372724/Ceftobiprole/1'>ceftobiprole</scene> and | ||
| + | ceftaroline – that have anti-MRSA activity have been developed. Ceftobiprole is able to | ||
| + | inhibit PBP2a because additional chemical groups at the | ||
| + | <scene name='37/372724/Ceftobiprole/7'>R2</scene> | ||
| + | position of the cephalosporin backbone are able to interact with additional amino acid | ||
| + | residues in PBP2a; specifically | ||
| + | <scene name='37/372724/Tyr446_and_met641_label/2'>Tyr446 and Met641</scene>. | ||
| + | As a result of ceftobiprole <scene name='37/372724/R2_interaction/4⅝'>tighter binding</scene> to PBP2a as highlighted in green , <scene name='37/372724/Ceftobiprole_in_cpk/1'>the medicine</scene>, shown as colors of the atom types ([[CPK]]), is able to more efficiently react with the serine active site residue and therefore inhibit the activity of PBP2a. | ||
| + | |||
| + | |||
| + | <scene name='37/372724/Medicine_interaction/3'>R2</scene> | ||
| + | <scene name='37/372724/Medicine_interaction/2'>Tyr446 and Met641</scene> | ||
| + | <scene name='37/372724/Medicine_interaction/1'>ceftobiprole</scene> | ||
| + | <scene name='37/372724/R2_interaction/6'>tighter binding</scene> | ||
Revision as of 17:38, 15 August 2013
| |||||||||||