This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Delta-endotoxin
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
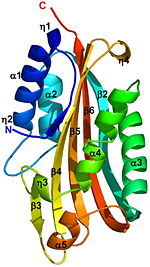
A remarkable similarity is observed between the structures of the endogenously cleaved Cyt2Ba <scene name='Cyt2Ba/Cyt2ba_monomer/2'>monomer</scene> <font color='gray'><b>(gray)</b></font> and the <scene name='Cyt2Ba/Alignment/2'>corresponding region</scene> <font color='red'><b>(red)</b></font> within the inactive protoxin <scene name='Cyt2Ba/Dimer/2'>dimer</scene> of Cyt2Aa ([[1cby]], monomers <font color='red'><b>A</b></font> and <font color='blue'><b>B</b></font> of Cyt2Aa shown <font color='red'><b>red</b></font> and <font color='blue'><b>blue</b></font>, respectively, the N- and C-termini are shown in spacefilling representation). Although, [[1cby]] is a 1 chain structure, the biological relevant molecule for [[1cby]] can be assembled from the contents of the deposited coordinates by the application of crystallographic symmetry operations to give a dimer. It can be [http://www.ebi.ac.uk/pdbe/pqs/macmol/1cby.mmol downloaded]. Each monomer of Cyt2Aa ([[1cby]]), consists of an additional β-strand at its N-terminus and an additional α-helix at its C-terminus compared to the cleaved Cyt2Ba. The <scene name='Cyt2Ba/Dimer_mesh/12'>dimer interface</scene> of Cyt2Aa is held together by the intertwined N-terminal strands from both monomers. The cleavage of Cyt2Aa <scene name='Cyt2Ba/Dimer_mes/1'>removes</scene> the N- and C-terminal segments, prevents dimer formation and releases an <scene name='Cyt2Ba/Monomer_toxin/4'> active toxin monomer</scene>. Similarly, in Cyt2Ba the proteolysis causes the removal of 34 amino acids at its N-terminus and 28 or 30 residues at its C-terminus forming the crystallized toxic monomer. | A remarkable similarity is observed between the structures of the endogenously cleaved Cyt2Ba <scene name='Cyt2Ba/Cyt2ba_monomer/2'>monomer</scene> <font color='gray'><b>(gray)</b></font> and the <scene name='Cyt2Ba/Alignment/2'>corresponding region</scene> <font color='red'><b>(red)</b></font> within the inactive protoxin <scene name='Cyt2Ba/Dimer/2'>dimer</scene> of Cyt2Aa ([[1cby]], monomers <font color='red'><b>A</b></font> and <font color='blue'><b>B</b></font> of Cyt2Aa shown <font color='red'><b>red</b></font> and <font color='blue'><b>blue</b></font>, respectively, the N- and C-termini are shown in spacefilling representation). Although, [[1cby]] is a 1 chain structure, the biological relevant molecule for [[1cby]] can be assembled from the contents of the deposited coordinates by the application of crystallographic symmetry operations to give a dimer. It can be [http://www.ebi.ac.uk/pdbe/pqs/macmol/1cby.mmol downloaded]. Each monomer of Cyt2Aa ([[1cby]]), consists of an additional β-strand at its N-terminus and an additional α-helix at its C-terminus compared to the cleaved Cyt2Ba. The <scene name='Cyt2Ba/Dimer_mesh/12'>dimer interface</scene> of Cyt2Aa is held together by the intertwined N-terminal strands from both monomers. The cleavage of Cyt2Aa <scene name='Cyt2Ba/Dimer_mes/1'>removes</scene> the N- and C-terminal segments, prevents dimer formation and releases an <scene name='Cyt2Ba/Monomer_toxin/4'> active toxin monomer</scene>. Similarly, in Cyt2Ba the proteolysis causes the removal of 34 amino acids at its N-terminus and 28 or 30 residues at its C-terminus forming the crystallized toxic monomer. | ||
The crystal structure of monomeric Cyt2Ba is the first structure of a [http://en.wikipedia.org/wiki/Toxicity toxic] form of the Cyt family. Its structure is [http://en.wikipedia.org/wiki/Homology_(biology) homologous] to the corresponding region of Cyt2Aa and to that of VVA2. This structural comparison shows that the toxicity of Cyt2Ba, Cyt2Aa and VVA2 is an inherent property of the monomer and not the result of secondary structure rearrangement upon cleavage. Solving the 3D structure of these proteins extends the knowledge of the cytolytic machinery of pore-forming toxins and helps in designing novel membrane-active cytotoxins. | The crystal structure of monomeric Cyt2Ba is the first structure of a [http://en.wikipedia.org/wiki/Toxicity toxic] form of the Cyt family. Its structure is [http://en.wikipedia.org/wiki/Homology_(biology) homologous] to the corresponding region of Cyt2Aa and to that of VVA2. This structural comparison shows that the toxicity of Cyt2Ba, Cyt2Aa and VVA2 is an inherent property of the monomer and not the result of secondary structure rearrangement upon cleavage. Solving the 3D structure of these proteins extends the knowledge of the cytolytic machinery of pore-forming toxins and helps in designing novel membrane-active cytotoxins. | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
== 3D Structures of δ-endotoxin == | == 3D Structures of δ-endotoxin == | ||
Revision as of 10:15, 20 November 2013
| |||||||||||
3D Structures of δ-endotoxin
Updated on 20-November-2013
3eb7 – BtCry8Ea1 – Bacillus thuringiensis
2rci – BtCytBa
2c9k – BtCry4A residues 68-679
1w99 - BtCry4Ba residues 84-641
4d8m - BtCry4Ba residues 112-698
1dlc - BtCryIiia residues 61-644
1ji6 - BtCryIiib residues 64-652
1cby - BtCytB residues 1-259
3ron – BtCry1aa
</StructureSection>
References
- Li J, Koni PA, Ellar DJ. Structure of the mosquitocidal delta-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996 Mar 22;257(1):129-52. PMID:8632451 doi:http://dx.doi.org/10.1006/jmbi.1996.0152
- Cohen S, Dym O, Albeck S, Ben-Dov E, Cahan R, Firer M, Zaritsky A. High-resolution crystal structure of activated Cyt2Ba monomer from Bacillus thuringiensis subsp. israelensis. J Mol Biol. 2008 Jul 25;380(5):820-7. Epub 2008 May 11. PMID:18571667 doi:10.1016/j.jmb.2008.05.010