We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 127
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
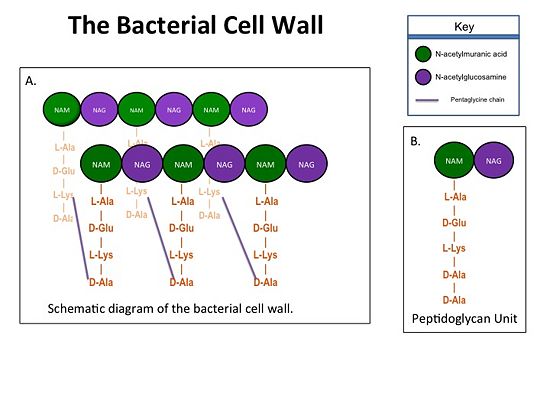
[[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate |550px]] | [[Image:Cell Wall 7 30 2013.jpg|thumb|alt= Alt text| Figure 1. A.Bacterial Cell Wall B.Peptidoglycan with D-Ala-D-Ala substrate |550px]] | ||
| - | |||
| - | == Structure of a Resistant Transpeptidase == | ||
| - | |||
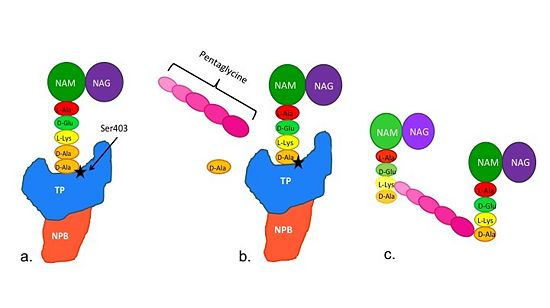
| - | Methicillin resistant Staphylococcus aureus (MRSA) is resistant to all β-lactams because it acquires an alternative PBP, PBP2a, that is not bound or inhibited by any β-lactams. PBP2a is composed of two domains: a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/25'>(NPB) </scene></font> and a <font color='dodgerblue'><b>transpeptidase <scene name='36/365380/4dki_cartoon/26'>(TP)</scene> binding domain </b></font>. The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain “sits” in the periplasm with its active site facing the inner surface of the cell wall. The active site contains <scene name='36/365380/Ser403/19'>a serine residue at position 403 (ser403)</scene> which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. | ||
| Line 34: | Line 30: | ||
== Structure of PBP2a, a B-lactam Resistant Transpeptidase== | == Structure of PBP2a, a B-lactam Resistant Transpeptidase== | ||
| - | Isolates of methicillin-resistant S. aureus (MRSA) are resistant to almost all currently available B-lactam because they have acquired an alternative PBP, PBP2a(encoded by the mecA gene), that is neither bound nor inhibited by B-lactams. PBP2a is composed of two domains:a a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/17'>(NPB) </scene></font> and a <font color='dodgerblue'><b>transpeptidase <scene name='36/365380/4dki_cartoon/18'>(TP)</scene> binding domain </b></font> | + | Isolates of methicillin-resistant S. aureus (MRSA) are resistant to almost all currently available B-lactam because they have acquired an alternative PBP, PBP2a(encoded by the mecA gene), that is neither bound nor inhibited by B-lactams. PBP2a is composed of two domains:a a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/17'>(NPB) </scene></font> and a <font color='dodgerblue'><b>transpeptidase <scene name='36/365380/4dki_cartoon/18'>(TP)</scene> binding domain </b></font>. The NPB domain of PBP2a is anchored in the cell membrane, while the TP domain resides in the periplasm with its active site facing the inner surface of the cell wall. The active site contains a serine residue at position 403 (Ser403) which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. |
Revision as of 21:20, 18 September 2013
| |||||||||||