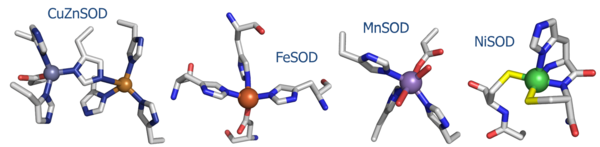
Molecular Playground/Nickel Superoxide Dismutase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
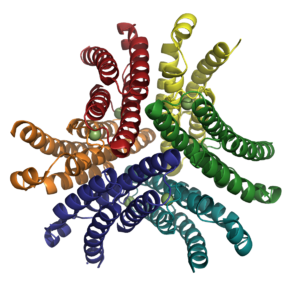
| - | -- | + | <StructureSection load='1q0m' size='350' side='right' caption='Ni-bound NiSOD (PDB ID: [[1q0m]]). The yellow/red molecules represent sulphates, the grey/red molecules represent acetic acid, and the green spheres represent the nickel ions bound by the nickel-hook region.' scene='' pspeed='8'> |
Nickel superoxide dismutase (NiSOD) is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts, Amherst Chemistry-Biology Interface Program] at Umass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | Nickel superoxide dismutase (NiSOD) is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts, Amherst Chemistry-Biology Interface Program] at Umass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | ||
| - | |||
| - | <Structure load='1q0m' size='350' frame='true' align='left' caption='Ni-bound NiSOD (PDB ID: [[1q0m]]). The yellow/red molecules represent sulphates, the grey/red molecules represent acetic acid, and the green spheres represent the nickel ions bound by the nickel-hook region.' scene='Insert optional scene name here' /> | ||
===Introduction=== | ===Introduction=== | ||
| Line 30: | Line 28: | ||
The [http://people.chem.umass.edu/mmaroney/research.html | Maroney Lab] is currently investigating the details of the NiSOD catalytic mechanism, including how the enzyme maintains its nickel active sites at 50% Ni(II)/Ni(III) equilibrium despite strong oxidation. In addition, efforts are currently underway to characterize a previously observed intermediate. | The [http://people.chem.umass.edu/mmaroney/research.html | Maroney Lab] is currently investigating the details of the NiSOD catalytic mechanism, including how the enzyme maintains its nickel active sites at 50% Ni(II)/Ni(III) equilibrium despite strong oxidation. In addition, efforts are currently underway to characterize a previously observed intermediate. | ||
| - | + | </StructureSection> | |
===References=== | ===References=== | ||
---- | ---- | ||
Revision as of 10:11, 29 December 2019
| |||||||||||
References
- ↑ Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147-59. PMID:1094908 doi:http://dx.doi.org/10.1146/annurev.bi.44.070175.001051
- ↑ Lee JW, Roe JH, Kang SO. Nickel-containing superoxide dismutase. Methods Enzymol. 2002;349:90-101. PMID:11912934
- ↑ Uedsemma, M., Tamm, T. Density-functional theory calculations of aqueous redox potentials of fourth-period transition metals. J. Phys. Chem. A. 2003 Aug 4;107:9997-10003. Epub 2003 Oct 23. 10.1021/jp0362741
- ↑ Bordo D, Matak D, Djinovic-Carugo K, Rosano C, Pesce A, Bolognesi M, Stroppolo ME, Falconi M, Battistoni A, Desideri A. Evolutionary constraints for dimer formation in prokaryotic Cu,Zn superoxide dismutase. J Mol Biol. 1999 Jan 8;285(1):283-96. PMID:9878406 doi:10.1006/jmbi.1998.2267
- ↑ Borgstahl GE, Parge HE, Hickey MJ, Beyer WF Jr, Hallewell RA, Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992 Oct 2;71(1):107-18. PMID:1394426
- ↑ Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML. Structure-function in Escherichia coli iron superoxide dismutase: comparisons with the manganese enzyme from Thermus thermophilus. Biochemistry. 1995 Feb 7;34(5):1646-60. PMID:7849024