Single stranded binding protein
From Proteopedia
| Line 1: | Line 1: | ||
| + | '''Single-stranded DNA-binding protein''', or SSB, binds to single-stranded regions of [[deoxyribonucleic acid|DNA]] to prevent premature [[Annealing_(biology)#Annealing|annealing]], to protect the single-stranded DNA from being digested by nucleases, and to remove secondary structure from the DNA to allow other enzymes to function effectively upon it. Single-stranded DNA is produced during all aspects of DNA metabolism: replication, recombination and repair. As well as stabilizing this single-stranded DNA, SSB proteins bind to and modulate the function of numerous proteins involved in all of these processes. | ||
| + | |||
| + | SSB proteins have been identified in organisms from viruses to humans. The only organisms known to lack them are [[Thermoproteales]], a group of extremophile [[archaea]], where they have been displaced by the protein [[ThermoDBP]]. While many phage and viral SSBs function as monomers and eukaryotes encode heterotrimeric [[Replication protein A| RPA]] (Replication Protein A), the best characterized SSB is that from the bacteria ''[[E. coli]]'' which, like most bacterial SSBs exists as a [[Tetrameric protein|tetramer]]. Active ''[[E. coli]]'' SSB is composed of four identical 19 kDa subunits. Binding of single-stranded DNA to the tetramer can occur in different "modes", with SSB occupying different numbers of DNA bases depending on a number of factors, including salt concentration. For example, the (SSB)<sub>65</sub> binding mode, in which approximately 65 nucleotides of DNA wrap around the SSB tetramer and contact all four of its subunits, is favoured at high salt concentrations ''in vitro''. At lower salt concentrations, the (SSB)<sub>35</sub> binding mode, in which about 35 nucleotides bind to only two of the SSB subunits, tends to form. Further work is required to elucidate the functions of the various binding modes ''in vivo''. | ||
| + | |||
| + | |||
== Sandbox Single Stranded DNA-Binding Protein (SSB)== | == Sandbox Single Stranded DNA-Binding Protein (SSB)== | ||
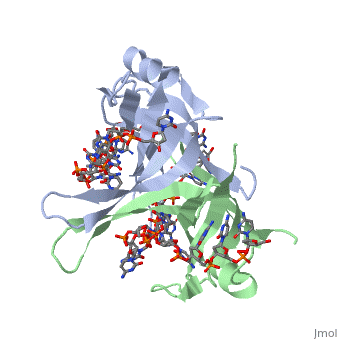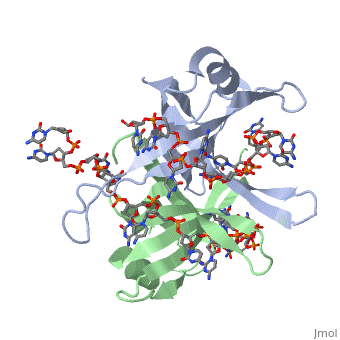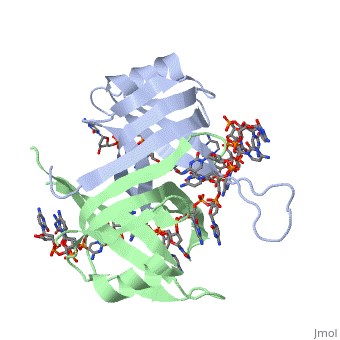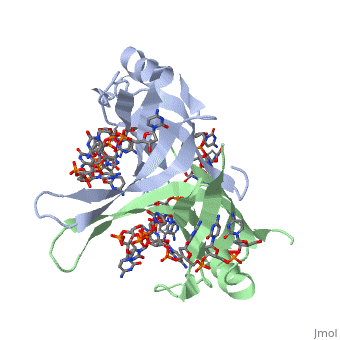
<StructureSection load='1eyg' size='500' side='right' frame='true' caption='Structure of Single Stranded DNA-Binding Protein bound to ssDNA (PDB entry [[1eyg]])' scene=''> | <StructureSection load='1eyg' size='500' side='right' frame='true' caption='Structure of Single Stranded DNA-Binding Protein bound to ssDNA (PDB entry [[1eyg]])' scene=''> | ||
| Line 4: | Line 9: | ||
The single stranded DNA binding protein (SSB) of E. coli plays an important role in three aspects of DNA metabolism – namely in replication, repair and recombination. During DNA replication, SSB molecules bind to the newly separated DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template <ref>PMID: 2087220</ref>. | The single stranded DNA binding protein (SSB) of E. coli plays an important role in three aspects of DNA metabolism – namely in replication, repair and recombination. During DNA replication, SSB molecules bind to the newly separated DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template <ref>PMID: 2087220</ref>. | ||
| - | |||
| - | |||
| - | |||
Revision as of 01:51, 2 November 2013
Single-stranded DNA-binding protein, or SSB, binds to single-stranded regions of DNA to prevent premature annealing, to protect the single-stranded DNA from being digested by nucleases, and to remove secondary structure from the DNA to allow other enzymes to function effectively upon it. Single-stranded DNA is produced during all aspects of DNA metabolism: replication, recombination and repair. As well as stabilizing this single-stranded DNA, SSB proteins bind to and modulate the function of numerous proteins involved in all of these processes.
SSB proteins have been identified in organisms from viruses to humans. The only organisms known to lack them are Thermoproteales, a group of extremophile archaea, where they have been displaced by the protein ThermoDBP. While many phage and viral SSBs function as monomers and eukaryotes encode heterotrimeric RPA (Replication Protein A), the best characterized SSB is that from the bacteria E. coli which, like most bacterial SSBs exists as a tetramer. Active E. coli SSB is composed of four identical 19 kDa subunits. Binding of single-stranded DNA to the tetramer can occur in different "modes", with SSB occupying different numbers of DNA bases depending on a number of factors, including salt concentration. For example, the (SSB)65 binding mode, in which approximately 65 nucleotides of DNA wrap around the SSB tetramer and contact all four of its subunits, is favoured at high salt concentrations in vitro. At lower salt concentrations, the (SSB)35 binding mode, in which about 35 nucleotides bind to only two of the SSB subunits, tends to form. Further work is required to elucidate the functions of the various binding modes in vivo.
Sandbox Single Stranded DNA-Binding Protein (SSB)
| |||||||||||
| |||||||||||
| |||||||||||
See Also
References
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
Proteopedia Page Contributors and Editors (what is this?)
Refayat Ahsen, Rachel Craig, Michal Harel, Alexander Berchansky