User:Andrew Wills/Sandbox 1
From Proteopedia
| Line 17: | Line 17: | ||
==Active Sites and Mechanism== | ==Active Sites and Mechanism== | ||
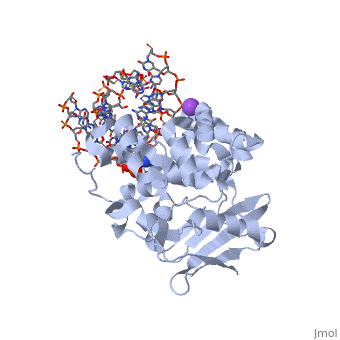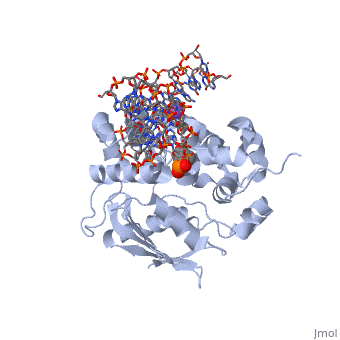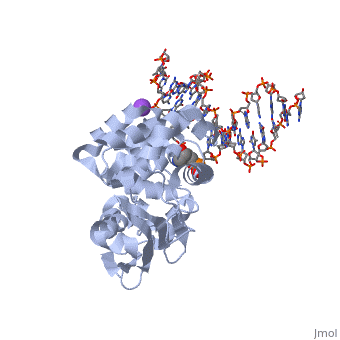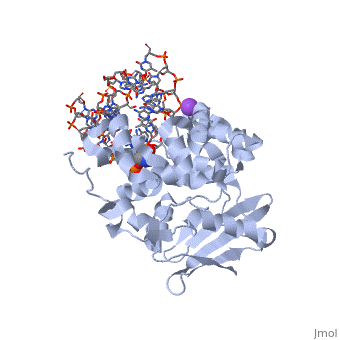
The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/2'>Leu 125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | The HhH segment of AlkA connects to the phosphodiester backbone of DNA by hydrogen bonding and <scene name='56/566536/Sodium_ion_interaction/1'>sodium ion interaction</scene>. The binding of the HhH segment to DNA stabilizes the damaged base and creates a 66 degree bend away from the protein that widens the minor groove of DNA.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Leu_125/2'>Leu 125</scene> fits into the minor groove between base pairs and allows the alkylated base to be flipped into the active site.<ref name="Hollis">Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print. </ref> | ||
| - | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/1'>DNA Binding Pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/1'>Trp272</scene> stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> Asp238 is essential for allowing the reaction to proceed, and points into the nonpolar pocket in order to allow stabilization of a carbocation intermediate. This stabilization is what allows the cleavage of the glycosidic bond on the damaged base.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> Mutation or inhibition of the Asp238 residue will prevent AlkA from performing base excision repair. | + | The AlkA active site is located where the second and third domains are separated by a deep nonpolar cleft that is lined with the aromatic side chains Phe18, Tyr273, Trp272, Tyr222, and Trp218.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> These side chains create a <scene name='56/566536/Binding_pocket/1'>DNA Binding Pocket</scene> for the alkylated base.<ref name="Labahn">Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.</ref> Once the damaged base is in the active site, <scene name='56/566536/Trp_272/1'>Trp272</scene> stabilizes the flipped out alkylated base in the binding pocket by aromatic ring-stacking interactions.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> <scene name='56/566536/Asp_238/1'>Asp238</scene> is essential for allowing the reaction to proceed, and points into the nonpolar pocket in order to allow stabilization of a carbocation intermediate. This stabilization is what allows the cleavage of the glycosidic bond on the damaged base.<ref name="Moe">Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print. </ref> Mutation or inhibition of the Asp238 residue will prevent AlkA from performing base excision repair. |
==DNA Interaction== | ==DNA Interaction== | ||
Revision as of 15:49, 6 November 2013
| |||||||||
| 1diz, resolution 2.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Non-Standard Residues: | |||||||||
| Activity: | DNA-3-methyladenine glycosylase II, with EC number 3.2.2.21 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
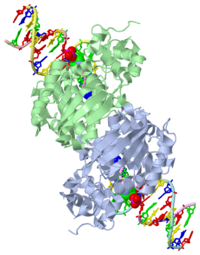
CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA
<nowiki>Insert non-formatted text here</nowiki>
General Function
1diz Escherichia coli 3 methyladenine DNA glycosylase II () is a DNA repair enzyme that initiates base excision repair for the removal of alkylated bases. Aflatoxin B1 is an example of a toxin that attacks guanosine at its N-7 atom to form alkylated bases[1], which prevent regulatory proteins from binding to DNA and blocks replicative polymerases.[2] AlkA initiates base excision repair by first locating and binding to the alkylated DNA. It then flips the affected base out of the DNA double helix and into the active site of the enzyme. Once in the active site, AlkA hydrolyzes the glycosidic bond to release the damaged base and leave the sugar phosphate backbone intact. This creates the AP site that is either devoid of a purine or pyridine. The AP site signals to other base excision repair enzymes to insert an undamaged nucleotide based on the undamaged complementary strand and seal the DNA.
| |||||||||||
See Also
References
- ↑ Berg, Jeremy, Tymoczko John, and Lubert Stryer. Biochemistry. 6th. New York: W.H. Freeman and Company, 2007. 806-808. Print.
- ↑ 2.0 2.1 2.2 Hollis, Thomas, Yoshitaka Ichikawa, and Tom Ellenberger. "DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA." EMBO Journal. 19.4 (2000): 758-766. Print.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Labahn, Jorg, Orlando Scharer, et al. "Structural Basis for the Excision Repair of Alkylation-Damaged DNA." Cell. 86.2 (1996): 321-329. Print.
- ↑ 4.0 4.1 4.2 4.3 Moe, E, D.R. Hall, et al. "Structure-function studies of an unusual 3-methyladenine DNA glycosylase II (AlkA) from Deinococcus radiodurans." Biological Crystallography. 68.6 (2012): 703-712. Print.