Sandbox Reserved 776
From Proteopedia
(→Dehaloperoxidase) |
(→Dehaloperoxidase) |
||
| Line 5: | Line 5: | ||
''' | ''' | ||
== Dehaloperoxidase == | == Dehaloperoxidase == | ||
| - | '''Dehaloperoxidase (DHP)''' from the marine annellid ''Amphitrite ornata'' is a dimeric enzyme weighing ~16 kDa with a heme prosthetic group and belongs to the globin family. DHP is consistent with the 'globin fold', having eight alpha helices; globins are reversible oxygen binding/transporting proteins (1). Although belonging to the globin superfamily, DHP has a biologically relevant peroxidase activity, the first known globin-peroxidase. It has previously been shown to dehalogenate trihalophenols to their corresponding dihaloquinones. Reactivity is initiated by use of H2O2 as co-substrate. [[Image:trihalophenol mech.jpg]] | + | '''Dehaloperoxidase (DHP)''' from the marine annellid ''Amphitrite ornata'' is a dimeric enzyme weighing ~16 kDa with a heme prosthetic group and belongs to the globin family. DHP is consistent with the 'globin fold', having eight alpha helices; globins are reversible oxygen binding/transporting proteins (1). Although belonging to the globin superfamily, DHP has a biologically relevant peroxidase activity, the first known globin-peroxidase. It has previously been shown to dehalogenate trihalophenols to their corresponding dihaloquinones. Reactivity is initiated by use of H2O2 as co-substrate. [[Image:trihalophenol mech.jpg]] More recent work has shown that DHP also reacts through a peroxygenase mechanism by substrate turnover with haloindoles and oxidase activity with indigo formation from 5-bromo-3-oxindole. |
Revision as of 20:07, 18 November 2013
|
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Dehaloperoxidase
Dehaloperoxidase (DHP) from the marine annellid Amphitrite ornata is a dimeric enzyme weighing ~16 kDa with a heme prosthetic group and belongs to the globin family. DHP is consistent with the 'globin fold', having eight alpha helices; globins are reversible oxygen binding/transporting proteins (1). Although belonging to the globin superfamily, DHP has a biologically relevant peroxidase activity, the first known globin-peroxidase. It has previously been shown to dehalogenate trihalophenols to their corresponding dihaloquinones. Reactivity is initiated by use of H2O2 as co-substrate. 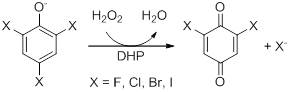 More recent work has shown that DHP also reacts through a peroxygenase mechanism by substrate turnover with haloindoles and oxidase activity with indigo formation from 5-bromo-3-oxindole.
More recent work has shown that DHP also reacts through a peroxygenase mechanism by substrate turnover with haloindoles and oxidase activity with indigo formation from 5-bromo-3-oxindole.
