Maureen E. Hill/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
*<scene name='Molecular_Playground/Caspase_Dynamics/1f1j/2'>Caspase-7 bound to suicide inhibitor/substrate mimic DEVD-CHO</scene>, trapping protein in active/substrate bound conformation. | *<scene name='Molecular_Playground/Caspase_Dynamics/1f1j/2'>Caspase-7 bound to suicide inhibitor/substrate mimic DEVD-CHO</scene>, trapping protein in active/substrate bound conformation. | ||
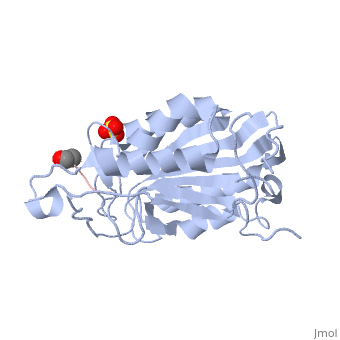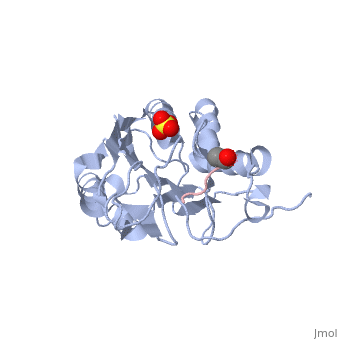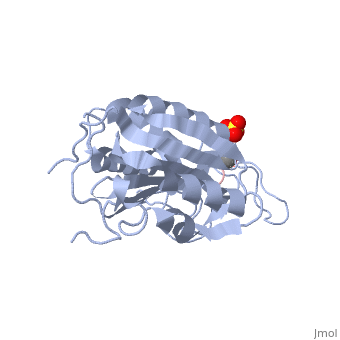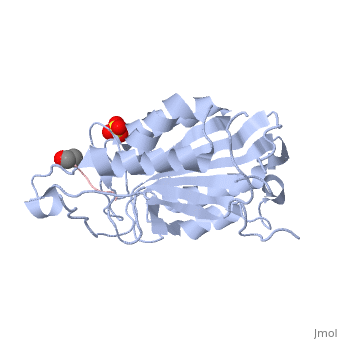
| + | <scene name='56/566502/Dica_bound_caspase_7/1'>Caspase-7 bound to allosteric inhibitor DICA</scene> at the dimer interface. The inhibitors bind to C290 within the dimer interface displacing Y223. The displacement of tyrosine from the active site conformation of the enzyme in turn forces R187 into a position that both physically blocks substrate binding, as well as move the active site cystine 186 which ultimately inactivate the enzyme. | ||
| + | |||
*<scene name='Molecular_Playground/Caspase_Dynamics/1shj-234234/1'>Caspase-7 bound to allosteric inhibitor DICA through CYS290</scene> trapping protein in a form incompatible with substrate binding. | *<scene name='Molecular_Playground/Caspase_Dynamics/1shj-234234/1'>Caspase-7 bound to allosteric inhibitor DICA through CYS290</scene> trapping protein in a form incompatible with substrate binding. | ||
*<scene name='Molecular_Playground/Caspase_Dynamics/Morph2/2'>Conformational change between substrate bound and substrate incompatible forms</scene> of Caspase-7. | *<scene name='Molecular_Playground/Caspase_Dynamics/Morph2/2'>Conformational change between substrate bound and substrate incompatible forms</scene> of Caspase-7. | ||
Revision as of 21:49, 2 December 2013
Caspase-7 Dynamics
| |||||||||||