Maureen E. Hill/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
*<scene name='Molecular_Playground/Caspase_Dynamics/1f1j/2'>Caspase-7 bound to suicide inhibitor/substrate mimic DEVD-CHO</scene>, trapping protein in active/substrate bound conformation. | *<scene name='Molecular_Playground/Caspase_Dynamics/1f1j/2'>Caspase-7 bound to suicide inhibitor/substrate mimic DEVD-CHO</scene>, trapping protein in active/substrate bound conformation. | ||
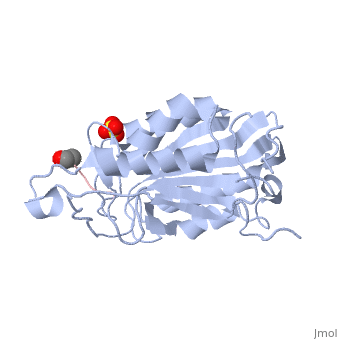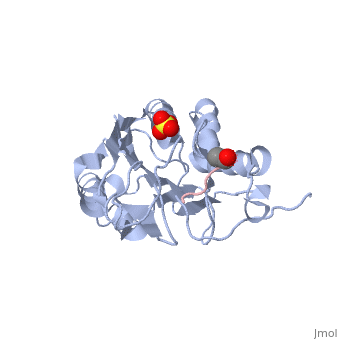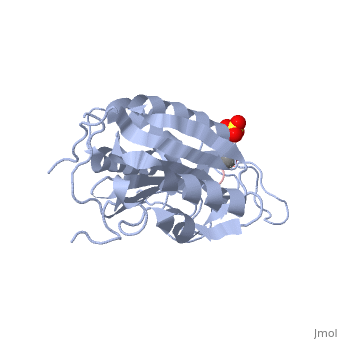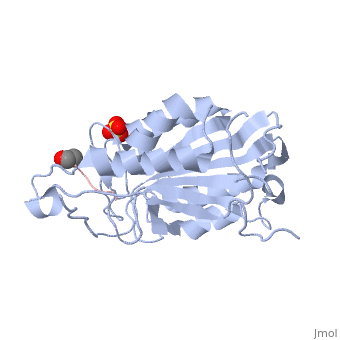
| - | <scene name='56/566502/Dica_bound_caspase_7/ | + | <scene name='56/566502/Dica_bound_caspase_7/2'>Caspase-7 bound to allosteric inhibitor DICA</scene> at the dimer interface. The allosteric inhibitor binds to C290 within the dimer interface displacing Y223. The displacement of tyrosine from the active site conformation of the enzyme forces R187 into a position that both physically blocks substrate binding, as well as, move the active site cystine 186. Ultimately, these conformational changes inactivate the enzyme. |
*<scene name='Molecular_Playground/Caspase_Dynamics/1shj-234234/1'>Caspase-7 bound to allosteric inhibitor DICA through CYS290</scene> trapping protein in a form incompatible with substrate binding. | *<scene name='Molecular_Playground/Caspase_Dynamics/1shj-234234/1'>Caspase-7 bound to allosteric inhibitor DICA through CYS290</scene> trapping protein in a form incompatible with substrate binding. | ||
*<scene name='Molecular_Playground/Caspase_Dynamics/Morph2/2'>Conformational change between substrate bound and substrate incompatible forms</scene> of Caspase-7. | *<scene name='Molecular_Playground/Caspase_Dynamics/Morph2/2'>Conformational change between substrate bound and substrate incompatible forms</scene> of Caspase-7. | ||
Revision as of 21:58, 2 December 2013
Caspase-7 Dynamics
| |||||||||||