Background
Caspases are cysteine-aspartate proteases that are responsible for the execution of apoptosis, also known as programmed cell death. Dysregulation of apoptosis has been linked to neurodegenerative disorders, including Alzheimer's and Huntington's, as well as inflammatory diseases and cancer.
The apoptotic caspases consist of two distinct classes: the initiators (caspase -2, -8, -9, and -10) and the executioners (caspase -3, -6, and -7). All caspases are synthesized as catalytically inactive zymogens that must undergo proteolytic cleavage to be activated during apoptosis. Initiator caspases are activated by upstream cellular events, which in turn cleave at distinct internal aspartate residues in the executioner caspases to remove the prodomain and separate the large and small subunits. The executioner caspases then cleave a wide range of targets within the cell that ultimately leads to cellular suicide.
Caspase-7 Structure
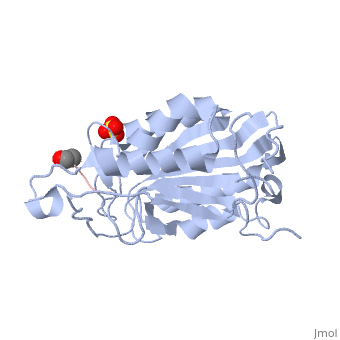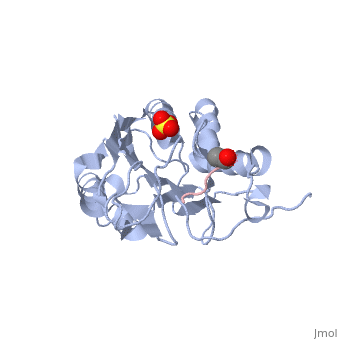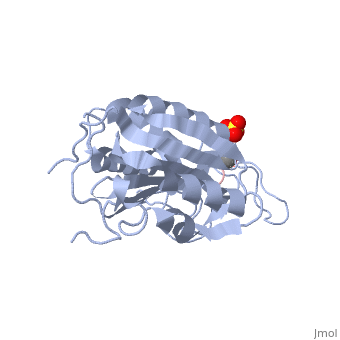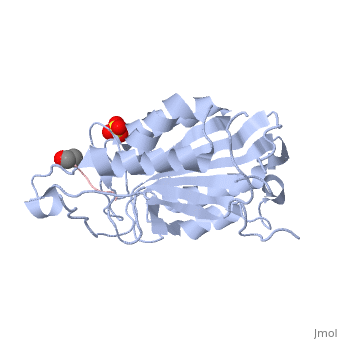
Caspases are crystallized as homodimers. Each monomer contains a large (~20 kDa) and a small (~10 kDa) subunit. 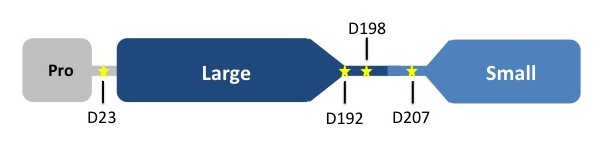
The is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the , the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. traps the protein an active/substrate bound conformation. Substrate binding forces L2' to move upward, creating a foundation beneath the L2 bundle. However, it has been shown that if caspase-7 were to bind an allosteric inhibitor, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide (FICA) or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide (DICA), at the dimer-interface cavity, the L2' loop would be locked in the down conformation, thereby inactivating the enzyme.
Forms of Caspase-7
, trapping protein in active/substrate bound conformation.
at the dimer interface. The allosteric inhibitor binds to C290 within the dimer interface displacing Y223. The displacement of tyrosine from the active site conformation of the enzyme forces R187 into a position that both physically blocks substrate binding, as well as, move the active site cystine 186. Ultimately, these conformational changes inactivate the enzyme.
trapping protein in a form incompatible with substrate binding.
of Caspase-7.