Sandbox Reserved 779
From Proteopedia
| Line 30: | Line 30: | ||
β-lactoglobulin (β-Lg) is a lipocalin, like plasma retinol-binding protein, so that ligand association was expected to make use of the central cavity in the protein.<ref>PMID:12054801</ref> | β-lactoglobulin (β-Lg) is a lipocalin, like plasma retinol-binding protein, so that ligand association was expected to make use of the central cavity in the protein.<ref>PMID:12054801</ref> | ||
| - | A cocrystallized β-Lg with palmitic acid ( | + | A cocrystallized β-Lg with palmitic acid (3D model 2), and the refined structure (R = 0.204, R free = 0.240 for 6,888 reflections to 2.5-Å resolution) reveals that the ligand binds in the central cavity in a manner similar to the binding of retinol to the related lipocalin, serum retinol-binding protein.<ref>PMID:9867826</ref> It is probably also involved in the transport of that molecule.<ref>PMID:15259212</ref>. |
====Transport Protein==== | ====Transport Protein==== | ||
| Line 42: | Line 42: | ||
===Dimer/Monomer=== | ===Dimer/Monomer=== | ||
| - | At physiological conditions, bovine b-lactoglobulin forms a dimer (Fig. 1), with each monomer consisting of 162 amino acid residues and characterized by a molecular mass of 18,350. Below pH 3, the dimer dissociates into monomers (Fig. | + | At physiological conditions, bovine b-lactoglobulin forms a dimer (Fig. 1), with each monomer consisting of 162 amino acid residues and characterized by a molecular mass of 18,350. Below pH 3, the dimer dissociates into monomers (Fig. 2) which preserve their native conformation.<ref>PMID:11734004</ref> |
| - | [[Image: | + | [[Image:BLG_Dimer_1BEB_Chain_A&B.png|thumb|right|320px|Figure 1. Bovine β-Lactoglobulin Dimer_[[1BEB]]]] |
Dimeric Lactoglobulin molecules exist in the open conformation at basic pH, whereas they exist in the closed conformation at acidic pH, after undergoing Tanford transition around neutral pH.<ref>PMID:17932936</ref> | Dimeric Lactoglobulin molecules exist in the open conformation at basic pH, whereas they exist in the closed conformation at acidic pH, after undergoing Tanford transition around neutral pH.<ref>PMID:17932936</ref> | ||
| Line 66: | Line 66: | ||
=='''Molecular mechanism of the Tanford transition'''== | =='''Molecular mechanism of the Tanford transition'''== | ||
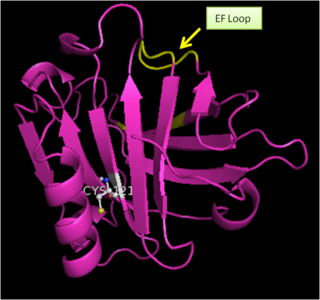
| - | The Tanford transition is a conformational change of bovine β-lactoglobulin occurring at around pH 7, identified originally on the basis of optical rotatory dispersion and the accessibility of a thiol group. X-ray analysis has suggested that a conformational change to the EF-loop is responsible for the Tanford transition, with the loop closing the hydrophobic cavity of the β-barrel of the β-LG molecule below pH 7 and flipping to open the cavity above pH 7.<ref>PMID:16368109</ref>Tanford transition is triggered by protonation of Glu89 exhibiting an anomalously high pKa value. The Tanford transition involves displacement/conformational change of the loop EF (residues 85 to 90) that acts as a lid which closes the protein interior/binding site below pH 7.3 and opens it at higher pH. The Tanford transition may involve some other structural changes as well. For example, the transition is accompanied by a change in the microenvironment of Tyr42 and causes an alteration in the relative orientation of monomers in the dimer by as much as 5 degrees, which breaks a number of intersubunit hydrogen bonds. It should be noted that all transitions that take place between pH 2 and pH 9 do not cause any appreciable changes in the native like β-barrel conformation of β-lactoglobulin. | + | The Tanford transition is a conformational change of bovine β-lactoglobulin occurring at around pH 7, identified originally on the basis of optical rotatory dispersion and the accessibility of a thiol group. X-ray analysis has suggested that a conformational change to the EF-loop (Fig. 2) is responsible for the Tanford transition, with the loop closing the hydrophobic cavity of the β-barrel of the β-LG molecule below pH 7 and flipping to open the cavity above pH 7.<ref>PMID:16368109</ref>Tanford transition is triggered by protonation of Glu89 exhibiting an anomalously high pKa value. The Tanford transition involves displacement/conformational change of the loop EF (residues 85 to 90) that acts as a lid which closes the protein interior/binding site below pH 7.3 and opens it at higher pH. The Tanford transition may involve some other structural changes as well. For example, the transition is accompanied by a change in the microenvironment of Tyr42 and causes an alteration in the relative orientation of monomers in the dimer by as much as 5 degrees, which breaks a number of intersubunit hydrogen bonds. It should be noted that all transitions that take place between pH 2 and pH 9 do not cause any appreciable changes in the native like β-barrel conformation of β-lactoglobulin. |
<ref>PMID:11734004</ref> | <ref>PMID:11734004</ref> | ||
| + | [[Image:BLG_monomer_showing_EF_Loop.png|thumb|right|320px|Figure 2. Bovine β-Lactoglobulin Monomer showing EF loop (colored yellow)]] | ||
The structures of the trigonal crystal form of bovine β-lactoglobulin variant A at pH 6.2, 7.1, and 8.2 have been determined by X-ray diffraction methods. The glutamate side chain of residue 89 is buried at pH 6.2 and becomes exposed at pH 7.1 and 8.2. This conformational change, involving the loop 85-90, provides a structural basis for a variety of pH-dependent chemical, physical, and spectroscopic phenomena, collectively known as the Tanford transition.<ref>PMID:9760236</ref>It was found that the stereochemical environment of Tyr42 changes significantly with pH variation between pH 7 and pH 8. This may provide a structural explanation for an as yet unexplained feature of the Tanford transition, namely the increase in exposure of a tyrosine residue.<ref>PMID:11168385</ref> | The structures of the trigonal crystal form of bovine β-lactoglobulin variant A at pH 6.2, 7.1, and 8.2 have been determined by X-ray diffraction methods. The glutamate side chain of residue 89 is buried at pH 6.2 and becomes exposed at pH 7.1 and 8.2. This conformational change, involving the loop 85-90, provides a structural basis for a variety of pH-dependent chemical, physical, and spectroscopic phenomena, collectively known as the Tanford transition.<ref>PMID:9760236</ref>It was found that the stereochemical environment of Tyr42 changes significantly with pH variation between pH 7 and pH 8. This may provide a structural explanation for an as yet unexplained feature of the Tanford transition, namely the increase in exposure of a tyrosine residue.<ref>PMID:11168385</ref> | ||
Revision as of 11:19, 5 December 2013
[[Image:Example.jpg]
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
β-Lactoglobulin
|
Contents |
β-Lactoglobulin
β-Lactoglobulin (β-LG) is the primary component of whey protein of cow’s milk with a concentration of 0.3 g/100 mL [1] and was first isolated in 1934 [2].
Under physiological conditions β-lactoglobulin exists as an equilibrium mixture of monomeric and dimeric forms. Its amino-acid sequence and 3-dimensional structure show that it is a member of lipocalin, a widely diverse family, most of which bind small hydrophobic ligands and thus may act as specific transporters, as does serum retinol binding protein. [3] β-Lactoglobulin is synthesized in mammary gland and secreted in milk. It causes an allergic reaction in human and is one of the causes of cow's milk allergy.
Bovine β-lactoglobulin (β-Lg) is a much studied and commercially important whey protein with an as yet undetermined function,although it is of obvious nutritional value. β-Lg binds a variety of ligands and by comparison of the general structures of these molecules together with several competition studies, it appears that there are at least 3 independent binding sites. In the absence of direct crystallographic evidence, a preliminary modelling study reveals that there is an internal cavity which can readily accommodate retinol in a manner similar to the related lipocalin, retinol-binding protein. On the outer surface, a solvent-accessible hydrophobic cleft runs between the 3-turn a-helix that is packed against the outer surface of the b-barrel. This cleft can accommodate fatty acids like palmitate and stearate. [4]
β-Lactoglobulin is a small protein, soluble in dilute salt solution as befits a globulin, with 162 amino acid residues (Mr ∼18,400)for each monomer that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand.
Lipocalin Proteins
β-Lactoglobulin belongs to the calycin superfamily and Lipocalin family. Lipocalins are typically small (160-180 residues in length), extracellular proteins sharing several common molecular recognition properties: the binding of small, principally hydrophobic molecules (such as retinol); binding to specific cell-surface receptors; and the formation of covalent and non-covalent complexes with other soluble macromolecules. Although they have been classified mainly as transport proteins [5]
The lipocalin family is a large and diverse family of proteins with functions varying from insect camouflage to small hydrophobic molecule transport typified by the serum retinol-binding protein [6] The crystal structures so far determined reveal the typical lipocalin to be an eight-stranded antiparallel β-barrel arranged to form a conical central calyx or cavity in which the hydrophobic ligand is located.[7]
Biological Function
Binding of variety of small hydrophobic molecules
|
Retinol and Palmitate Binding
β-lactoglobulin (β-Lg) is a lipocalin, like plasma retinol-binding protein, so that ligand association was expected to make use of the central cavity in the protein.[8] A cocrystallized β-Lg with palmitic acid (3D model 2), and the refined structure (R = 0.204, R free = 0.240 for 6,888 reflections to 2.5-Å resolution) reveals that the ligand binds in the central cavity in a manner similar to the binding of retinol to the related lipocalin, serum retinol-binding protein.[9] It is probably also involved in the transport of that molecule.[10].
Transport Protein
.................
Structure of β-Lactoglobulin
Residues and secondary structures
β-Lactoglobulin consists of 162 amino acid residues (18 kDa), containing two disulfide bonds (Cys 66–Cys 160 and Cys 106–Cys 119) and a free thiol (Cys 121). Structures of βLG have been reported by several groups with X-ray crystallography [19–21] and solution NMR [29,40,41].[11]It is a predominantly β-sheet protein. The β-barrel, or so called calyx, is conical and is made of two β-sheets: the B–D strands and N-terminal half of the A strand (denoted AN) form one sheet, and the E–H strands and C-terminal half of the A strand (denoted AC) form the other. On the outer surface of the β-barrel, between the G and H strands, is the 3-turn α-helix.The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG,are generally quite short, whereas those at the open end, AB, CD, EF,and GH, are significantly longer and more flexible. In the calyx,there is a large central cavity which is surrounded by hydrophobic residues and is accessible to solvent. This cavity provides the principal ligand-binding site.
Dimer/Monomer
At physiological conditions, bovine b-lactoglobulin forms a dimer (Fig. 1), with each monomer consisting of 162 amino acid residues and characterized by a molecular mass of 18,350. Below pH 3, the dimer dissociates into monomers (Fig. 2) which preserve their native conformation.[12]

Dimeric Lactoglobulin molecules exist in the open conformation at basic pH, whereas they exist in the closed conformation at acidic pH, after undergoing Tanford transition around neutral pH.[13]
Variants
Genetically, β-lactoglobulin may exist as one of several variants, among which the variants A and B are the most abundant. The A and B variants of the protein differ from each other by amino acid residues at positions Asp64 (Gly64 in variant B) and Val118 (Ala118 in variant B). These differences in primary structure render the two variants slightly different with respect to isoelectric point, solubility, self-association properties, as well as pressure and temperature stability.[14]
However, the structural characteristics of the A and B variants of bovine b-lactoglobulin are virtually indistinguishable. In its native state, β-lactoglobulin is a predominantly β-sheet protein containing nine b-strands and three a-helices. The core of the protein is formed by a flattened b-barrel (a calyx) composed of eight antiparallel b-strands (A to H).[15]
Ligands
Most lipocalins bind small hydrophobic molecules within the central cup or calyx. The true function of β-Lg is unknown, but it has been suggested that it is involved in the transport of retinol and/or fatty acids [8,50]. It binds retinol with a higher affinity than does RBP [51] and, as with RBP, specific binding of retinol to β-Lg has been observed in the small intestine of the neonatal calf [3]. The structure of RBP with retinol bound within the hydrophobic calyx has been solved [2] and retinol was successfully modelled into our previous β-Lg structure [3]. β-Lg contains two tryptophans, Trp19 and Trp61, and their fluorescence is altered when retinol is bound [51].[16]
Co-crystallized β-Lg with palmitic acid, and the refined structure (R = 0.204, R free = 0.240 for 6,888 reflections to 2.5-Å resolution) reveals that the ligand binds in the central cavity in a manner similar to the binding of retinol to the related lipocalin, serum retinol-binding protein. The carboxyl group binds to both Lys-60 and Lys-69 at the entrance to the cavity. The hydrophobic tail stretches in an almost fully extended conformation into the center of the protein.[17]
hydrophobic ligands and lactose ligands [18]
Active sites
βLG contains two tryptophan residues, Trp 19 on the A strand and Trp 61 on the C strand. The former is buried in the hydrophobic core whereas the latter is exposed to the solvent in the native structure, making them useful probes for monitoring site-specific conformational changes.[19]
In addition, studies on the monomer–dimer equilibrium [30,32,42,43] and the reactivity of the thiol group of Cys121 which deeply buried between the α-helix and H strand [44–48] revealed other important properties of β-LG.[20] the stability of the structure also depend so heavily upon the external loop around residue 64 or the β strand with the free thiol.[21]
Molecular mechanism of the Tanford transition
The Tanford transition is a conformational change of bovine β-lactoglobulin occurring at around pH 7, identified originally on the basis of optical rotatory dispersion and the accessibility of a thiol group. X-ray analysis has suggested that a conformational change to the EF-loop (Fig. 2) is responsible for the Tanford transition, with the loop closing the hydrophobic cavity of the β-barrel of the β-LG molecule below pH 7 and flipping to open the cavity above pH 7.[22]Tanford transition is triggered by protonation of Glu89 exhibiting an anomalously high pKa value. The Tanford transition involves displacement/conformational change of the loop EF (residues 85 to 90) that acts as a lid which closes the protein interior/binding site below pH 7.3 and opens it at higher pH. The Tanford transition may involve some other structural changes as well. For example, the transition is accompanied by a change in the microenvironment of Tyr42 and causes an alteration in the relative orientation of monomers in the dimer by as much as 5 degrees, which breaks a number of intersubunit hydrogen bonds. It should be noted that all transitions that take place between pH 2 and pH 9 do not cause any appreciable changes in the native like β-barrel conformation of β-lactoglobulin. [23]
The structures of the trigonal crystal form of bovine β-lactoglobulin variant A at pH 6.2, 7.1, and 8.2 have been determined by X-ray diffraction methods. The glutamate side chain of residue 89 is buried at pH 6.2 and becomes exposed at pH 7.1 and 8.2. This conformational change, involving the loop 85-90, provides a structural basis for a variety of pH-dependent chemical, physical, and spectroscopic phenomena, collectively known as the Tanford transition.[24]It was found that the stereochemical environment of Tyr42 changes significantly with pH variation between pH 7 and pH 8. This may provide a structural explanation for an as yet unexplained feature of the Tanford transition, namely the increase in exposure of a tyrosine residue.[25]
Implications or possible application
β-LG interaction with hydrophobic molecules and with other proteins, and its sensitivity to chemical, thermal and baric denaturation, all with a view to establishing relationships among structure, properties and functionality [26]
Antioxidant Nature
In the dairy industry, bovine milk is frequently heated for pasteurization (62.5°C for 30 min) and sterilization. This heating process may induce oxidative losses of proteins, unsaturated lipids, vitamins, active enzymes, and immunological factors. Cross-linking the free thiol groups of β-LG by heating (100 degrees C for 2 min), or chemically modifying the β-LG by carboxymethylation to block the thiol groups resulted in a substantial loss of antioxidant activity. The data suggest that Cys-121 plays an essential role in the antioxidant nature of β-LG.Because β-LG is extremely sensitive to thermal denaturation, to maintain its antioxidant nature, dairy products consumed daily should not be overheated in order to maintain its antioxidant nature.[27]
Transport molecule
vehicle to transport molecules to the gut
External Sources
....
Other β-Lactoglobulin related 3D Structures and complexes
2q2m - Bovine β-Lactoglobulin Native (Fig. 4)
1b8e - Crystal structure of the Bovine β-Lactoglobulin (Isoforms A and B) in orthorombic space group
1qg5 - Crystal structure of the Bovine β-Lactoglobulin (Isoforms A)
1beb - Bovine β-Lactoglobulin, Lattice X
1cj5 - Bovine β-Lactoglobulin A
1gx8 - Bovine β-Lactoglobulin complexed with Retinol, Trigonal Lattice Z
1gx9 - Bovine β-Lactoglobulin complexed with Retinoic acid, Trigonal Lattice Z
1gxa - Bovine β-Lactoglobulin complexed with Retinol and Palmitic acid, Trigonal Lattice Z
1b0o - Bovine β-Lactoglobulin complexed with Palmitate, Lattice Z
1bsy 2blg 3blg - Structural Basis of the Tanford Transitioon of Bovine β-Lactoglobulin from crystal structures at 3 pH values
References
- ↑ BELL K, MCKENZIE HA. BETA-LACTOGLOBULINS. Nature. 1964 Dec 26;204:1275-9. PMID:14254409
- ↑ http://www.jbc.org/content/104/2/359.citation
- ↑ Kontopidis G, Holt C, Sawyer L. Invited review: beta-lactoglobulin: binding properties, structure, and function. J Dairy Sci. 2004 Apr;87(4):785-96. PMID:15259212 doi:http://dx.doi.org/10.3168/jds.S0022-0302(04)73222-1
- ↑ http://www.sciencedirect.com/science/article/pii/S0958694698000211
- ↑ Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):9-24. PMID:11058743
- ↑ http://www.biochemj.org/bj/318/bj3180001.htm
- ↑ Newcomer ME, Jones TA, Aqvist J, Sundelin J, Eriksson U, Rask L, Peterson PA. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451-4. PMID:6540172
- ↑ Kontopidis G, Holt C, Sawyer L. The ligand-binding site of bovine beta-lactoglobulin: evidence for a function? J Mol Biol. 2002 May 10;318(4):1043-55. PMID:12054801 doi:10.1016/S0022-2836(02)00017-7
- ↑ Wu SY, Perez MD, Puyol P, Sawyer L. beta-lactoglobulin binds palmitate within its central cavity. J Biol Chem. 1999 Jan 1;274(1):170-4. PMID:9867826
- ↑ Kontopidis G, Holt C, Sawyer L. Invited review: beta-lactoglobulin: binding properties, structure, and function. J Dairy Sci. 2004 Apr;87(4):785-96. PMID:15259212 doi:http://dx.doi.org/10.3168/jds.S0022-0302(04)73222-1
- ↑ Sakurai K, Konuma T, Yagi M, Goto Y. Structural dynamics and folding of beta-lactoglobulin probed by heteronuclear NMR. Biochim Biophys Acta. 2009 Jun;1790(6):527-37. doi: 10.1016/j.bbagen.2009.04.003., Epub 2009 Apr 10. PMID:19362581 doi:http://dx.doi.org/10.1016/j.bbagen.2009.04.003
- ↑ Taulier N, Chalikian TV. Characterization of pH-induced transitions of beta-lactoglobulin: ultrasonic, densimetric, and spectroscopic studies. J Mol Biol. 2001 Dec 7;314(4):873-89. PMID:11734004 doi:http://dx.doi.org/10.1006/jmbi.2001.5188
- ↑ Vijayalakshmi L, Krishna R, Sankaranarayanan R, Vijayan M. An asymmetric dimer of beta-lactoglobulin in a low humidity crystal form-Structural changes that accompany partial dehydration and protein action. Proteins. 2007 Oct 11;71(1):241-249. PMID:17932936 doi:10.1002/prot.21695
- ↑ Taulier N, Chalikian TV. Characterization of pH-induced transitions of beta-lactoglobulin: ultrasonic, densimetric, and spectroscopic studies. J Mol Biol. 2001 Dec 7;314(4):873-89. PMID:11734004 doi:http://dx.doi.org/10.1006/jmbi.2001.5188
- ↑ Taulier N, Chalikian TV. Characterization of pH-induced transitions of beta-lactoglobulin: ultrasonic, densimetric, and spectroscopic studies. J Mol Biol. 2001 Dec 7;314(4):873-89. PMID:11734004 doi:http://dx.doi.org/10.1006/jmbi.2001.5188
- ↑ Brownlow S, Morais Cabral JH, Cooper R, Flower DR, Yewdall SJ, Polikarpov I, North AC, Sawyer L. Bovine beta-lactoglobulin at 1.8 A resolution--still an enigmatic lipocalin. Structure. 1997 Apr 15;5(4):481-95. PMID:9115437
- ↑ Wu SY, Perez MD, Puyol P, Sawyer L. beta-lactoglobulin binds palmitate within its central cavity. J Biol Chem. 1999 Jan 1;274(1):170-4. PMID:9867826
- ↑ Dominguez-Ramirez L, Del Moral-Ramirez E, Cortes-Hernandez P, Garcia-Garibay M, Jimenez-Guzman J. beta-Lactoglobulin's Conformational Requirements for Ligand Binding at the Calyx and the Dimer Interphase: a Flexible Docking Study. PLoS One. 2013 Nov 8;8(11):e79530. doi: 10.1371/journal.pone.0079530. PMID:24255705 doi:http://dx.doi.org/10.1371/journal.pone.0079530
- ↑ Sakurai K, Konuma T, Yagi M, Goto Y. Structural dynamics and folding of beta-lactoglobulin probed by heteronuclear NMR. Biochim Biophys Acta. 2009 Jun;1790(6):527-37. doi: 10.1016/j.bbagen.2009.04.003., Epub 2009 Apr 10. PMID:19362581 doi:http://dx.doi.org/10.1016/j.bbagen.2009.04.003
- ↑ Sakurai K, Konuma T, Yagi M, Goto Y. Structural dynamics and folding of beta-lactoglobulin probed by heteronuclear NMR. Biochim Biophys Acta. 2009 Jun;1790(6):527-37. doi: 10.1016/j.bbagen.2009.04.003., Epub 2009 Apr 10. PMID:19362581 doi:http://dx.doi.org/10.1016/j.bbagen.2009.04.003
- ↑ Brownlow S, Morais Cabral JH, Cooper R, Flower DR, Yewdall SJ, Polikarpov I, North AC, Sawyer L. Bovine beta-lactoglobulin at 1.8 A resolution--still an enigmatic lipocalin. Structure. 1997 Apr 15;5(4):481-95. PMID:9115437
- ↑ Sakurai K, Goto Y. Dynamics and mechanism of the Tanford transition of bovine beta-lactoglobulin studied using heteronuclear NMR spectroscopy. J Mol Biol. 2006 Feb 17;356(2):483-96. Epub 2005 Dec 1. PMID:16368109 doi:http://dx.doi.org/10.1016/j.jmb.2005.11.038
- ↑ Taulier N, Chalikian TV. Characterization of pH-induced transitions of beta-lactoglobulin: ultrasonic, densimetric, and spectroscopic studies. J Mol Biol. 2001 Dec 7;314(4):873-89. PMID:11734004 doi:http://dx.doi.org/10.1006/jmbi.2001.5188
- ↑ Qin BY, Bewley MC, Creamer LK, Baker HM, Baker EN, Jameson GB. Structural basis of the Tanford transition of bovine beta-lactoglobulin. Biochemistry. 1998 Oct 6;37(40):14014-23. PMID:9760236 doi:10.1021/bi981016t
- ↑ Oliveira KM, Valente-Mesquita VL, Botelho MM, Sawyer L, Ferreira ST, Polikarpov I. Crystal structures of bovine beta-lactoglobulin in the orthorhombic space group C222(1). Structural differences between genetic variants A and B and features of the Tanford transition. Eur J Biochem. 2001 Jan;268(2):477-83. PMID:11168385
- ↑ http://www.sciencedirect.com/science/article/pii/S0958694698000211
- ↑ Liu HC, Chen WL, Mao SJ. Antioxidant nature of bovine milk beta-lactoglobulin. J Dairy Sci. 2007 Feb;90(2):547-55. PMID:17235131 doi:http://dx.doi.org/10.3168/jds.S0022-0302(07)71538-2