User:Alisha, Deepa, Pamiz/Sandbox 1
From Proteopedia
(→Introduction) |
(→H+/K+-ATPase) |
||
| Line 6: | Line 6: | ||
== H+/K+-ATPase == | == H+/K+-ATPase == | ||
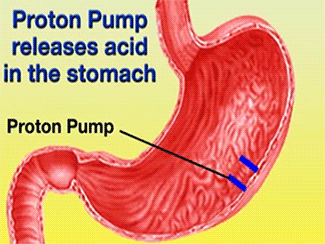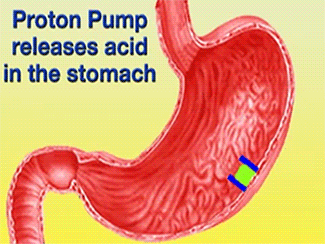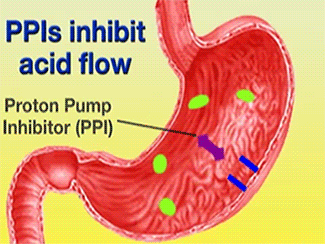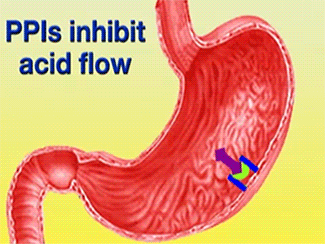
| - | + | The H+/K+-ATPase pump is part of the P-type ATPase family located within the cytoplasmic membrane of resting parietal cells (Figure 1); powered through ATP hydrolysis, the ATPase is translocated to the canalicular membrane and begins to pump cytoplasmic H+ into the canalicular space, in exchange for extracellular K+ ions. It is a pro-drug that is protonated twice in the acidic environment of the parietal cell to form the active inhibitor, sulfenamide which forms disulfide bonds with Cys 813 and Cys 892 on the α subunit of the H+/K+-ATPase.3[[Image:cell.jpg|300px|right|thumb|] '''Cartoon representation of Parietal Cell''' Image courtesy of ...]] Targeting this enzyme using PPIs is the most effective therapeutic control agent of gastric acid secretion.3 | |
| - | The structure of H+/K+-ATPase is an α,β-heterodimeric enzyme, where the catalytic site is present in the α subunit (Figure 2).7 Four transmembrane segments (TM4, TM5, TM6, and TM8) are located in the α subunit and contain the ion binding region of the enzyme | + | The structure of H+/K+-ATPase is an α,β-heterodimeric enzyme, where the catalytic site is present in the α subunit (Figure 2).7 Four transmembrane segments (TM4, TM5, TM6, and TM8) are located in the α subunit and contain the ion binding region of the enzyme.7 Binding of ions and ATP to these domains will induce movements in the membrane domain that catalyze ion displacement.7 |
H+/K+-ATPase is part of the P-type ATPase enzyme family, and transports cations across the membrane.5 The pump goes against the H+ concentration gradient found in the stomach and is powered through ATP hydrolysis.5 The inorganic Pi produced drives a conformational change in the enzyme and allows release of H+ into the highly acidic environment.5 The enzyme catalyzes this reaction by changing conformation states between E1 and E2 (Scheme 1).6 The cytoplasm facing E1 conformational state has a high affinity for H+ ions, while the lumen facing E2 has a high affinity for K+ ions.6 The reaction begins when a hydronium ion binds to the enzyme on the cytoplasmic surface.6 MgATP will phosphorylate the enzyme at an Asp386 residue in a DKTG amino acid sequence to form the E1~Pi H+ intermediate.6 E1 undergoes a conformational change to form E2, where the ion site is exposed and H+ is released at a pH ~ 1.0.7 Extracellular K+ ions then bind to the same exposed region and the enzyme dephosphorylates.7 An occluded form of the enzyme (trapped) is formed once K+ ions bind; the enzyme de-occludes, reforms the E1 complex, and K+ is released.7 | H+/K+-ATPase is part of the P-type ATPase enzyme family, and transports cations across the membrane.5 The pump goes against the H+ concentration gradient found in the stomach and is powered through ATP hydrolysis.5 The inorganic Pi produced drives a conformational change in the enzyme and allows release of H+ into the highly acidic environment.5 The enzyme catalyzes this reaction by changing conformation states between E1 and E2 (Scheme 1).6 The cytoplasm facing E1 conformational state has a high affinity for H+ ions, while the lumen facing E2 has a high affinity for K+ ions.6 The reaction begins when a hydronium ion binds to the enzyme on the cytoplasmic surface.6 MgATP will phosphorylate the enzyme at an Asp386 residue in a DKTG amino acid sequence to form the E1~Pi H+ intermediate.6 E1 undergoes a conformational change to form E2, where the ion site is exposed and H+ is released at a pH ~ 1.0.7 Extracellular K+ ions then bind to the same exposed region and the enzyme dephosphorylates.7 An occluded form of the enzyme (trapped) is formed once K+ ions bind; the enzyme de-occludes, reforms the E1 complex, and K+ is released.7 | ||
Revision as of 03:18, 6 December 2013
Introduction
H+/K+-ATPase
The H+/K+-ATPase pump is part of the P-type ATPase family located within the cytoplasmic membrane of resting parietal cells (Figure 1); powered through ATP hydrolysis, the ATPase is translocated to the canalicular membrane and begins to pump cytoplasmic H+ into the canalicular space, in exchange for extracellular K+ ions. It is a pro-drug that is protonated twice in the acidic environment of the parietal cell to form the active inhibitor, sulfenamide which forms disulfide bonds with Cys 813 and Cys 892 on the α subunit of the H+/K+-ATPase.3 Targeting this enzyme using PPIs is the most effective therapeutic control agent of gastric acid secretion.3The structure of H+/K+-ATPase is an α,β-heterodimeric enzyme, where the catalytic site is present in the α subunit (Figure 2).7 Four transmembrane segments (TM4, TM5, TM6, and TM8) are located in the α subunit and contain the ion binding region of the enzyme.7 Binding of ions and ATP to these domains will induce movements in the membrane domain that catalyze ion displacement.7
H+/K+-ATPase is part of the P-type ATPase enzyme family, and transports cations across the membrane.5 The pump goes against the H+ concentration gradient found in the stomach and is powered through ATP hydrolysis.5 The inorganic Pi produced drives a conformational change in the enzyme and allows release of H+ into the highly acidic environment.5 The enzyme catalyzes this reaction by changing conformation states between E1 and E2 (Scheme 1).6 The cytoplasm facing E1 conformational state has a high affinity for H+ ions, while the lumen facing E2 has a high affinity for K+ ions.6 The reaction begins when a hydronium ion binds to the enzyme on the cytoplasmic surface.6 MgATP will phosphorylate the enzyme at an Asp386 residue in a DKTG amino acid sequence to form the E1~Pi H+ intermediate.6 E1 undergoes a conformational change to form E2, where the ion site is exposed and H+ is released at a pH ~ 1.0.7 Extracellular K+ ions then bind to the same exposed region and the enzyme dephosphorylates.7 An occluded form of the enzyme (trapped) is formed once K+ ions bind; the enzyme de-occludes, reforms the E1 complex, and K+ is released.7
The interaction between Esomeprazole and H+/K+-ATPase has not yet been crystallized.15 However, data obtained from the crystallized structure of a SCH28080-ATPase provides structural and binding site information.16 SCH28080 is a competitive K+ inhibitor that interacts with the enzyme’s Phe126 residue and prevents disulfide bond formation between Omeprazole and Cys813.16 This suggests that SCH28080 and Esomeprazole have mutually exclusive inhibitions showing an overlapping binding site.16 The crystallized structure of SCH28080-ATPase shows the same luminal-open (E2) conformation as Esomeprazole, and is the first and only crystallized evidence of PPI-ATPase binding site and conformational change.16 Using this information, the SCH28080-ATPase crystallized structure provides evidence of the two binding sites, Cys813 and Cys892.15,16 The binding pocket, as proposed using SCH28080 includes the following residues: Glu936, Lys791, Glu795, Cys813, Cys892, Phe126, and Glu822 (Figure 5). The negatively charged residues within the TM domains are important for K+ binding and are conserved in all P-type ATPases.16 The SCH28080 model shows that electrostatic and hydrophobic factors affect drug-enzyme interaction.16
![] Cartoon representation of Parietal Cell Image courtesy of ...](/wiki/images/thumb/e/e8/Cell.jpg/300px-Cell.jpg)