User:Alisha, Deepa, Pamiz/Sandbox 1
From Proteopedia
(→H+/K+-ATPase) |
(→Drug-Molecule Interaction) |
||
| Line 23: | Line 23: | ||
The interaction between Esomeprazole and H+/K+-ATPase has not yet been crystallized.15 Data obtained from the crystallized structure of a '''SCH28080-ATPase''' provides structural and binding site information.16 SCH28080 is a competitive K+ inhibitor that interacts with the enzyme’s Phe126 residue and prevents disulfide bond formation between Omeprazole and Cys813, suggesting mutually exclusive inhibitions and an overlapping binding site.16 The crystallized structure of SCH28080-ATPase shows the same luminal-open (E2) conformation as Esomeprazole, and is the first and only crystallized evidence of PPI-ATPase binding site and conformational change.16 Using this information, the SCH28080-ATPase crystallized structure provides evidence of the two binding sites, Cys813 and Cys892.15,16 | The interaction between Esomeprazole and H+/K+-ATPase has not yet been crystallized.15 Data obtained from the crystallized structure of a '''SCH28080-ATPase''' provides structural and binding site information.16 SCH28080 is a competitive K+ inhibitor that interacts with the enzyme’s Phe126 residue and prevents disulfide bond formation between Omeprazole and Cys813, suggesting mutually exclusive inhibitions and an overlapping binding site.16 The crystallized structure of SCH28080-ATPase shows the same luminal-open (E2) conformation as Esomeprazole, and is the first and only crystallized evidence of PPI-ATPase binding site and conformational change.16 Using this information, the SCH28080-ATPase crystallized structure provides evidence of the two binding sites, Cys813 and Cys892.15,16 | ||
| - | The binding pocket, as proposed using SCH28080 includes the following residues: Glu936, Lys791, Glu795, Cys813, Cys892, Phe126, and Glu822 (Figure 5). The negatively charged residues within the TM domains are important for K+ binding and are conserved in all P-type ATPases.16 The SCH28080 model shows that electrostatic and hydrophobic factors affect drug-enzyme interaction.16[[Image:pymol.jpg|300px|left|thumb| '''Crystalized Structure of H+/K+-ATPase ''' TM helices 5,6,7,8 are each highlighted in a different color; the majority of the A & B domain within the enzyme, non-catalytic portions of the enzyme, and SCH28080 have been omitted. The figure depicts the proposed binding sites of the crystallized structure of H+/K+-ATPase. Cys813 and Cys892 are highlighted as the two disulfide bond formation sites. Cys 813 is found between TM5 and TM6 domains while Cys892 is between TM7 and TM8 domains.14 Sulfenamide will interact with these two residues and form disulfide bonds. ]][[Image:pymol_2.jpg|300px| | + | The binding pocket, as proposed using SCH28080 includes the following residues: Glu936, Lys791, Glu795, Cys813, Cys892, Phe126, and Glu822 (Figure 5). The negatively charged residues within the TM domains are important for K+ binding and are conserved in all P-type ATPases.16 The SCH28080 model shows that electrostatic and hydrophobic factors affect drug-enzyme interaction.16[[Image:pymol.jpg|300px|left|thumb| '''Crystalized Structure of H+/K+-ATPase ''' TM helices 5,6,7,8 are each highlighted in a different color; the majority of the A & B domain within the enzyme, non-catalytic portions of the enzyme, and SCH28080 have been omitted. The figure depicts the proposed binding sites of the crystallized structure of H+/K+-ATPase. Cys813 and Cys892 are highlighted as the two disulfide bond formation sites. Cys 813 is found between TM5 and TM6 domains while Cys892 is between TM7 and TM8 domains.14 Sulfenamide will interact with these two residues and form disulfide bonds. ]][[Image:pymol_2.jpg|300px|right|thumb| '''Binding pocket of SCH28080-ATPase complex''' PDB image obtained from the RCSB Protein Data Bank.15 Image created using PyMol™ Molecular Graphics System. Glu936 (orange), Glu822 (orange), Lys791 (yellow), Glu 795 (orange), Phe126 (gray), Cys892 (light blue), and Cys813 (light blue) are proposed to be part of Esomeprazole’s binding cavity.15,16 ]] |
| - | + | ||
== Esomeprazole Resistance == | == Esomeprazole Resistance == | ||
Revision as of 04:31, 6 December 2013
Contents |
Introduction
Esomeprazole is the (S) enantiomer of Omeprazole. Esomperazole is a Proton Pump Inhibitor (PPI) that binds to H+/K+-ATPase and inhibits the secretion of gastric acid from the parietal cells into the lumen of the stomach. Esomeprazole’s commercial brand name, Nexium, is used to treat Gastro-esophageal Reflux Disease (GERD), peptic and gastric ulcers, and Zollinger-Ellison syndrome.[CITATION??]
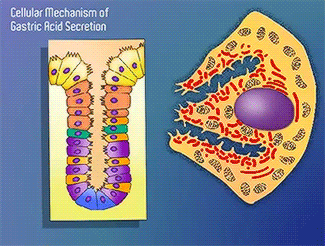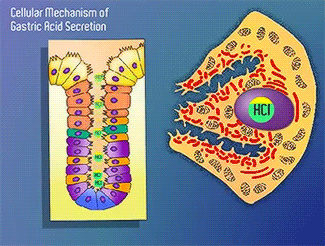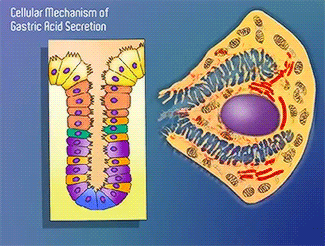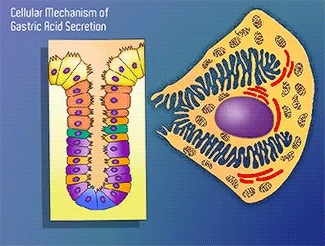
H+/K+-ATPase
The H+/K+-ATPase pump is part of the P-type ATPase family located within the cytoplasmic membrane of resting parietal cells (Figure 1); powered through ATP hydrolysis, the ATPase is translocated to the canalicular membrane and begins to pump cytoplasmic H+ into the canalicular space, in exchange for extracellular K+ ions. It is a pro-drug that is protonated twice in the acidic environment of the parietal cell to form the active inhibitor, sulfenamide which forms disulfide bonds with Cys 813 and Cys 892 on the α subunit of the H+/K+-ATPase.3 Targeting this enzyme using PPIs is the most effective therapeutic control agent of gastric acid secretion.3 Esomeprazole is an α,β-heterodimeric enzyme, where the catalytic site is present in the α subunit(Figure 2).7 Transmembrane segments TM4, TM5, TM6, and TM8, are located in the α subunit and contain the ion binding region of the enzyme.7 Binding of ions and ATP to these domains will induce movements in the membrane domain that catalyze ion displacement.7
Conformational Change and Signaling Pathway of H+/K+-ATPase
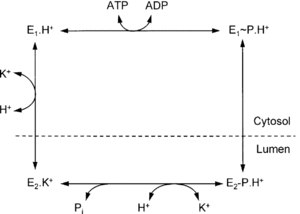

Esomeprazole Mechanism
Esomeprazole is protonated twice within the acidic environment, and forms the active inhibitor—achiral sulfenamide at pKa of 1 (Scheme 2).11
Drug-Molecule Interaction
The interaction between Esomeprazole and H+/K+-ATPase has not yet been crystallized.15 Data obtained from the crystallized structure of a SCH28080-ATPase provides structural and binding site information.16 SCH28080 is a competitive K+ inhibitor that interacts with the enzyme’s Phe126 residue and prevents disulfide bond formation between Omeprazole and Cys813, suggesting mutually exclusive inhibitions and an overlapping binding site.16 The crystallized structure of SCH28080-ATPase shows the same luminal-open (E2) conformation as Esomeprazole, and is the first and only crystallized evidence of PPI-ATPase binding site and conformational change.16 Using this information, the SCH28080-ATPase crystallized structure provides evidence of the two binding sites, Cys813 and Cys892.15,16
The binding pocket, as proposed using SCH28080 includes the following residues: Glu936, Lys791, Glu795, Cys813, Cys892, Phe126, and Glu822 (Figure 5). The negatively charged residues within the TM domains are important for K+ binding and are conserved in all P-type ATPases.16 The SCH28080 model shows that electrostatic and hydrophobic factors affect drug-enzyme interaction.16Esomeprazole Resistance
Resistance to treatment with PPIs, including Esomeprazole, has been speculated among Barrett’s Esophagus (BE) patients who did not indicate any symptomatic improvement after being placed on a standard PPI drug dose.17 No contributory mutations causing PPI resistance have been found.17 It is speculated that the high acid exposure in BE patients may be due to “reflux diathesis” rather than resistance to gastric acid secretion.18
Other possible reasons for PPI failure include Helicobacter pylori infection, rapid metabolism, and bioavailability; reasons of clinical significance include delayed gastric emptying and visceral hypersensitivity17 . More studies need to be conducted to understand the mechanisms underlying the development of resistance to PPIs.17
