Sandbox Reserved 777
From Proteopedia
| Line 17: | Line 17: | ||
== Mechanism of Action == | == Mechanism of Action == | ||
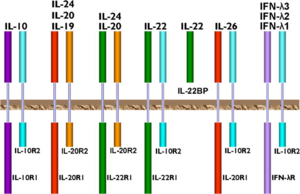
| - | Most hematopoietic cells express the IL-10 transmembrane receptors, IL-10R1 and IL-10R2. While IL-10R1 is specific only for IL-10 cytokine, IL-10R2 is also part of the receptor complexes for other ligands in the IL-10 family of cytokines. | + | [[Image:IL-10.receptors.png|300px|left|thumb|Receptors of the IL-10 family of cytokines.<ref name= "zdanov" />]]Most hematopoietic cells express the IL-10 transmembrane receptors, IL-10R1 and IL-10R2. While IL-10R1 is specific only for IL-10 cytokine, IL-10R2 is also part of the receptor complexes for other ligands in the IL-10 family of cytokines. The affinity for IL-10 is higher for the IL-10R complex than for IL-10R1 alone.<ref name="sabat" /> IL-10 is a pleiotropic cytokine in that it has an effect on many different immune cells, including T-cells, B-cells, macrophages and mast cells.<ref name="dewaal" /> When IL-10 binds the extracellular domain of the IL-10R1 receptor, two tyrosine-kinases, JAK1 and Tyk2 become phosphorylated, which in turn phosphorylate the internal domain of IL-10R1. This then binds the transcription factor STAT3, which becomes phosphorylated, dimerizes, and moves into the nucleus. In the nucleus, STAT3 binds to the promoters of various genes, resulting in decreased expression of inflammatory cytokines such as IFNγ, TNFα, and GM-CSF.<ref>Thompson Reuters. Web. 16Nov2013. <http://pathwaymaps.com/maps/531/></ref> IL-10R2 acts as an accessory subunit for signaling, rather than for ligand binding. It recruits the Tyk2 tyrosine kinase into the signaling complex described above.<scene name='56/564053/Il_10-il_10r1/3'>IL-10 bound to IL-10R1</scene> forms a tetramer, made up of the dimeric two-domain IL-10 molecule and two IL-10R1 receptors.<ref name="moore" />The IL-10R1 receptor in this image is shown in dark blue. |
== Implications or Possible Application == | == Implications or Possible Application == | ||
Revision as of 19:23, 6 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Contents |
Introduction
|
Interleukin 10 (IL-10) belongs to a class of proteins called cytokines. Cytokines are chemical messengers that are produced by and act on cells of the immune system.[1] It is also part of the IL-10 family of cytokines, which also includes IL-19, IL-20, IL-22, IL-24 and IL-26. This grouping was made based on similarities in structure, function, and location of the encoding genes.[2] IL-10 has also previously been called cytokine synthesis inhibitory factor (CSIF) because of its anti-inflammatory properties and ability to inhibit the production of other cytokines.[3] The ability to limit the immune response to pathogens is crucial to preventing self-inflicted damage to the host. This cytokine is expressed in many eukaryotic hosts, as well as some viruses. A quick protein BLAST[4] of the human IL-10 sequence reveals homology with IL-10 of many other primate species, as well as that found in bats, horses, elephants, rodents, sheep, cats and birds. The remainder of this page will focus on human IL-10.
Structure
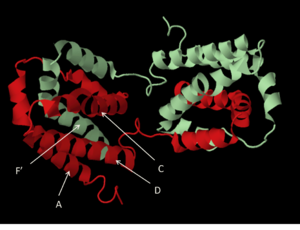
The cytokine structure is an intercalated dimer of two identical polypeptide chains, each 178 amino acids in length.[7] The monomers are made up of six amphipathic helices, with two helices of one monomer interacting with four of the other monomer, creating two distinct domains. A four-helix bundle (see image, at left) is made up of helices A, C, D and F’ (and A’, C’ D’ and F). This feature is a signature element of all helical cytokines.[8] The structure is stabilized by (shown in yellow)formed between the first and third cysteine residues and the second and fourth cysteine residues (Cys12 with Cys108, and Cys62 with Cys114). Reduction of the disulfide bonds in recombinant IL-10 has been shown to reduce helical content measured by circular dichroism and results in a lack of in vitro biological activity.[9]Protein Expression
IL-10 is a broadly expressed cytokine, usually in response to a stimulus. The gene is located on chromosome 1 and encodes a 178 amino acid peptide. It is translated by cells of the adaptive immune system through a variety of T- and B-cells, as well as by the innate immune system through dendritic cells, natural killer cells, mast cells, macrophages, eosinophils and neutrophils.[10] The exact signaling pathway that leads to IL-10 expression in most immune cells is unclear; however, it is known that the extra-cellular signal regulated kinase (ERK) cascade is one pathway activated in macrophages and myeloid dendritic cells that does lead to IL-10 expression.[10]
Mechanism of Action
Implications or Possible Application
Interleukin-10 plays a role in a number of diseases, particularly those with an autoimmune facet. One example of this is Crohn’s disease, a chronic inflammatory bowel condition in which the immune system attacks the gastrointestinal tract. It has been shown that IL-10 production in patients with severe Crohn’s is down regulated.[12] In a phase I clinical trial,[13] patients were treated with a Lactobacillus species that had been genetically modified to encode human IL-10. Patients reported a decrease in disease activity with no serious adverse events. On the other hand, patients with Systemic Lupus Erythematosus (SLE) have been found to have circulating IL-10 levels 9-fold higher than normal controls. It is believed that increased production of IL-10 stimulates production of hyperactive B-cells, which in turn leads to excessive autoantibody production. These autoantibodies trigger inflammatory responses that cause tissue damage. In fact, mouse models of SLE have shown that administration of anti-IL-10 monoclonal antibodies reduces morbidity due to disease, while giving additional IL-10 speeds the progression of disease.[14]
Viral IL-10
Viral homologs to Il-10 have been found in Epstein-Barr virus (EBV), human cytomegalovirus (hCMV) and multiple other members of the Herpesvirales and Poxviridae families of viruses. These viruses likely acquired IL-10 encoding sequences from their hosts and, when expressed, produce vIL-10 with enough similarity to host IL-10 that they can bind to IL-10R1 and start the signaling cascade. The result is the suppression of immune functions and enhancement of infection.[15] The EBV IL-10 monomer is shown , with the vIL-10 in light blue and the IL-10R1 receptor shown in dark blue
References
- ↑ National Institute of Allergy and Infectious Diseases, ”Immune Cells and their Products: Cytokines”. Web. 16Nov2013 <http://www.niaid.nih.gov/topics/immuneSystem/immuneCells/Pages/cytokines.aspx>
- ↑ 2.0 2.1 Sabat, Robert. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010; 21:315–324
- ↑ 3.0 3.1 de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries, JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991 Nov; 174(5):1209-1220
- ↑ Basic Local Alignment Search Tool (BLAST), http://blast.ncbi.nlm.nih.gov/Blast.cgi
- ↑ pyMOL molecular visualization sytem (Windows version), http://www.pymol.org
- ↑ RCSB Protein Data Bank, http://www.rcsb.org
- ↑ 7.0 7.1 Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the Interleukin-10 receptor. Annu. Rev. Immunol. 2001.19:683-765
- ↑ 8.0 8.1 Zdanov, A. Structural analysis of cytokines comprising the IL-10 family. Cytokine Growth Factor Rev. 2010; 21:325-330
- ↑ Windsor WT, Syto R, Tsarbopoulos A, et al. Disulfide bond assignments and secondary structure analysis of human and murine interleukin 10. Biochemistry. 1993; 32:8807-8815
- ↑ 10.0 10.1 Saraiva M and O’Garra A. The regulation of IL-10 production by immune cells. Nature Reviews Immunology. 2010 Mar; 10:170-181
- ↑ Thompson Reuters. Web. 16Nov2013. <http://pathwaymaps.com/maps/531/>
- ↑ Correa I, Veny M, Esteller M, Piqué JM, Yagüe J, Panés J, Salas A. Defective IL-10 production in severe phenotypes of Crohn’s disease. J Leukoc Biol. 2009 May; 85(5):896-903
- ↑ Braat H, Rottiers P, Hommes DW, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006 Jun; 4(6):754-759
- ↑ Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukins-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev. 2002 Aug-Oct; 13(4-5):403-412
- ↑ Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol. 2009 Oct; 83(19):9618-9629