Molecular Playground/Pcr H
From Proteopedia
| Line 14: | Line 14: | ||
'''References:''' | '''References:''' | ||
| - | <references/><ref>PMID:21770428</ref> | + | <references/PMID:21770428> |
| + | <ref>PMID:21770428</ref> | ||
Revision as of 21:45, 20 December 2013
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground
|
PcrH
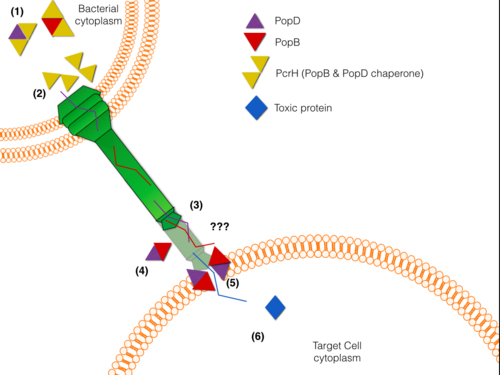
Pseudomonas aeruginosa is a bacterium widely found in the environment, nonetheless is capable of infecting patients with a weak immune system. P. aeruginosa has been linked to more than 65% of cases of human infections that exhibit great resistance to antibiotics; moreover, is the predominant cause of mortality in cystic fibrosis patients. As many Gram-negative pathogens, P. aeruginosa is armed with a sophisticated Type III Secretion System (T3SS) used to inject toxic proteins into human host cells. T3SS has been shown to have an important role in disease and pathogenicity of this bacterium. The T3SS is structured like a syringe that protrudes from the bacterial membrane towards the target cell membrane.PcrH crystal structure has been solved carrying a homologous sequence that is present in these translocator proteins. PcrH has a (Histidine and Arginine residues shown in yellow) where it binds to a (VELNAP) present in both PopB and PopD (9 residue peptide (rainbow) shown bound to Chain A). PcrH's characteristic fold is composed of Tetratricopeptide Repeats (TPR repeats). TPR repeats consist of a 34 amino-acid sequence motif that folds into two antiparallel alpha-helices that serve as interaction modules and multi-protein complex mediators. In this case, PcrH from Pseudomonas aeruginosa consist of three . The first Tpr motif is represented in red, the second in green and the third in cyan color, also an additional alpha-helix is represented in blue. This fold is also present in Eukaryotic co-chaperons such as HOP(Heat shock protein Organizing Protein). The VELNAP sequence motif binding is displayed as three hydrophobic pockets in the concave side of the TPR domain receive three hydrophobic residues within the CBM present in the synthetic peptide. This interaction keeps them in a metastable non oligomeric state in the cytosol, thus protecting them from degradation and aggregation. It is also believed that PcrH has an active role in delivering PopB and PopD to the basal body of the T3S system, where an ATPse unfolds and secretes the translocators PopB and PopD.
Given its key role in molecular pathogenesis PcrH is a potential target for drug design.
References:
<references/PMID:21770428>
[1]
Proteopedia Page Contributors and Editors (what is this?)
Carolina Morell-Perez, Yuzhou Tang, Michal Harel, Fabian Romano, Alexander Berchansky, Luis E Ramirez-Tapia