We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 828
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
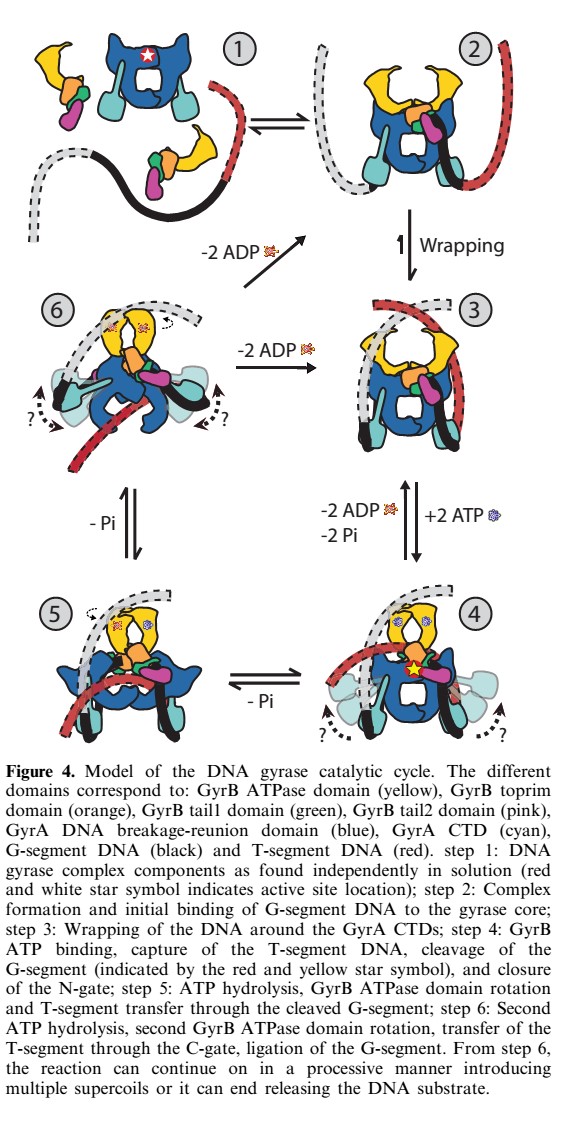
| - | Gyrase is the '''only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA''' in order to remove positive supercoils. It '''catalyses the hydrolysis of two phosphodiester bonds in a DNA segment''' (called G segment). Then, thanks to '''ATP dependant conformation changes''' it enables the passage of another segment (the T segment) through the break, and then religates the broken segment. Gyrase acts prior to the replication (before the replication fork) or other mecanisms requiring loose DNA. | + | Gyrase is the '''only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA''' (see [http://en.wikipedia.org/wiki/DNA_supercoil DNA Supercoil]) in order to remove positive supercoils. It '''catalyses the hydrolysis of two phosphodiester bonds in a DNA segment''' (called G segment). Then, thanks to '''ATP dependant conformation changes''' it enables the passage of another segment (the T segment) through the break, and then religates the broken segment. Gyrase acts prior to the replication (before the replication fork) or other mecanisms requiring loose DNA. |
'''In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.''' | '''In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.''' | ||
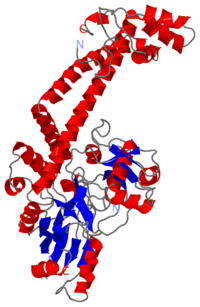
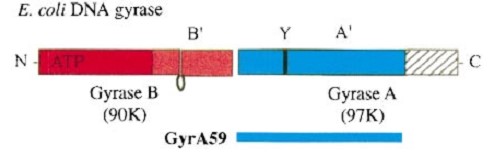
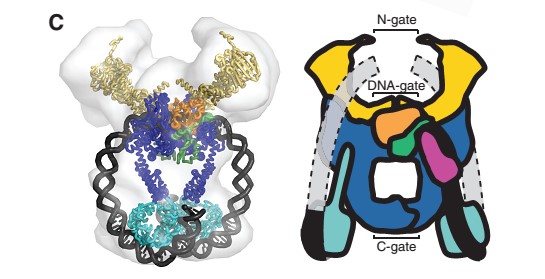
| - | Gyrase is coded by two differents contiguous genes gyrA and GgyrB as it is a 350 kDa | + | Gyrase is coded by two differents contiguous genes gyrA and GgyrB as it is a 350 kDa A<sub>2</sub>B<sub>2</sub> heterotetramers of two A proteins and two B proteins. |
The A protein breaks and religates DNA . The B protein has ATPase activity | The A protein breaks and religates DNA . The B protein has ATPase activity | ||
| Line 36: | Line 36: | ||
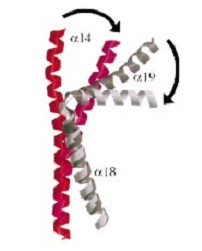
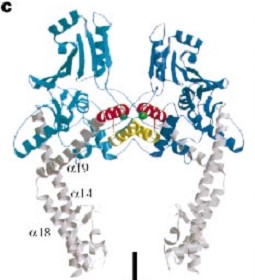
| - | The three connecting helices ( | + | The three connecting helices (α14,α18 and α19) adopt very different conformations, leading to large quaternary movements involving a single hinge-point within the helices and rigid body movements of |
the head fragments. | the head fragments. | ||
| Line 77: | Line 77: | ||
The gyrase structure reveals a '''new cluster of conserved residues''', juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. '''This cluster may form the active site of the breakage–reunion reaction''', with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA. | The gyrase structure reveals a '''new cluster of conserved residues''', juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. '''This cluster may form the active site of the breakage–reunion reaction''', with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA. | ||
| + | The main reaction catalysed by the gyrase is the DNa cleavage. It requires the energy of ATP-Hydrolisis. 2 ATP are hydrolised per reaction (2 B-subunits in the gyrase)so that the linking number (Lk) changes in step of 2. | ||
| + | The DNA supercoiling reaction requires in addition to ATP, a divalent cation such as Mg<sup>2+</sup>, and is stimulated in the presence of spermidine. | ||
| + | Therefore, the gyrase could also catalyse the following reaction : | ||
| + | * | ||
| + | |||
| + | |||
==See Also== | ==See Also== | ||
*[[Gyrase|Gyrase]] | *[[Gyrase|Gyrase]] | ||
Revision as of 20:12, 29 December 2013
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||