We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
m |
|||
| Line 37: | Line 37: | ||
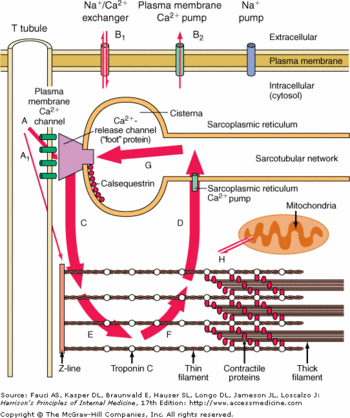
Each monomere of CASQ2 can bind between 18 to 50 Ca2+. The Ca2+ ions bind to two or more acidic amino acids like <scene name='56/568018/Glu/2'>Glutamate</scene> or <scene name='56/568018/Asp/3'>Aspartate</scene>. These amino acids are mainly outside the CASQ2 or in the C-terminal region. It had been shown that Ca2+ binds to an Asp-rich region on the C-terminal domain. <!-- METTRE DU VERT MAIS LE CT N'EST PAS DISPONIBLE cf: http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=2VAF&bionumber=1 -->When CASQ2 form homooligomers, Ca2+ can bind in the electronegative pocket due to the front-to-front form and back-to-back form.<ref name="The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000)">http://www.sciencedirect.com/science/article/pii/S0014579300022468</ref> | Each monomere of CASQ2 can bind between 18 to 50 Ca2+. The Ca2+ ions bind to two or more acidic amino acids like <scene name='56/568018/Glu/2'>Glutamate</scene> or <scene name='56/568018/Asp/3'>Aspartate</scene>. These amino acids are mainly outside the CASQ2 or in the C-terminal region. It had been shown that Ca2+ binds to an Asp-rich region on the C-terminal domain. <!-- METTRE DU VERT MAIS LE CT N'EST PAS DISPONIBLE cf: http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=2VAF&bionumber=1 -->When CASQ2 form homooligomers, Ca2+ can bind in the electronegative pocket due to the front-to-front form and back-to-back form.<ref name="The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000)">http://www.sciencedirect.com/science/article/pii/S0014579300022468</ref> | ||
| - | Ca2+ is not the only ion which can bind to the CASQ2. One of them is Mg2+. The affinity is for Mg2+ is lower than the affinity for Ca2+ however the number of Ca2+ decrease. Another ion is H+. When the pH is low, more H+ will bind to the acidic amino acids and they can not bind Ca2+ anymore.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)"> | + | Ca2+ is not the only ion which can bind to the CASQ2. One of them is Mg2+. The affinity is for Mg2+ is lower than the affinity for Ca2+ however the number of Ca2+ decrease. Another ion is H+. When the pH is low, more H+ will bind to the acidic amino acids and they can not bind Ca2+ anymore.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">PMID:15050380</ref> |
<!-- Source: Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15050380 --> | <!-- Source: Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15050380 --> | ||
Revision as of 16:33, 2 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004 May;85(1):33-69. PMID:15050380 doi:http://dx.doi.org/10.1016/j.pbiomolbio.2003.07.001
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 http://www.ncbi.nlm.nih.gov/pubmed/15731387