This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
m |
m |
||
| Line 35: | Line 35: | ||
== Calcium Binding == | == Calcium Binding == | ||
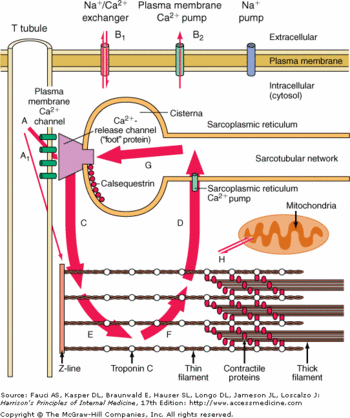
| - | Each monomere of CASQ2 can bind between 18 to 50 Ca2+. The Ca2+ ions bind to two or more acidic amino acids like <scene name='56/568018/Glu/2'>Glutamate</scene> or <scene name='56/568018/Asp/3'>Aspartate</scene>. These amino acids are mainly outside the CASQ2 or in the C-terminal region. It had been shown that Ca2+ binds to an Asp-rich region on the C-terminal domain. <!-- METTRE DU VERT MAIS LE CT N'EST PAS DISPONIBLE cf: http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=2VAF&bionumber=1 -->When CASQ2 form homooligomers, Ca2+ can bind in the electronegative pocket due to the front-to-front form and back-to-back form.<ref name="The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000)">http://www.sciencedirect.com/science/article/pii/S0014579300022468</ref> | + | Each monomere of CASQ2 can bind between 18 to 50 Ca2+. The Ca2+ ions bind to two or more acidic amino acids like <scene name='56/568018/Glu/2'>Glutamate</scene> or <scene name='56/568018/Asp/3'>Aspartate</scene>. These amino acids are mainly outside the CASQ2 or in the C-terminal region. It had been shown that Ca2+ binds to an Asp-rich region on the C-terminal domain. <!-- METTRE DU VERT MAIS LE CT N'EST PAS DISPONIBLE cf: http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=2VAF&bionumber=1 -->When CASQ2 form homooligomers, Ca2+ can bind in the electronegative pocket due to the front-to-front form and back-to-back form.<ref name="The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000)">The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000) http://www.sciencedirect.com/science/article/pii/S0014579300022468</ref> |
Ca2+ is not the only ion which can bind to the CASQ2. One of them is Mg2+. The affinity is for Mg2+ is lower than the affinity for Ca2+ however the number of Ca2+ decrease. Another ion is H+. When the pH is low, more H+ will bind to the acidic amino acids and they can not bind Ca2+ anymore.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">PMID:15050380</ref> | Ca2+ is not the only ion which can bind to the CASQ2. One of them is Mg2+. The affinity is for Mg2+ is lower than the affinity for Ca2+ however the number of Ca2+ decrease. Another ion is H+. When the pH is low, more H+ will bind to the acidic amino acids and they can not bind Ca2+ anymore.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">PMID:15050380</ref> | ||
Revision as of 16:42, 2 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000) http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004 May;85(1):33-69. PMID:15050380 doi:http://dx.doi.org/10.1016/j.pbiomolbio.2003.07.001
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005 May;88(5):3444-54. Epub 2005 Feb 24. PMID:15731387 doi:http://dx.doi.org/10.1529/biophysj.104.051441