Sandbox Reserved 815
From Proteopedia
| Line 21: | Line 21: | ||
| - | ==Structure== | + | ==Structure of 3HAF domain of Human Prion== |
---- | ---- | ||
| Line 69: | Line 69: | ||
| - | == | + | ==Biological Application== |
| + | PYM | ||
| - | + | ==Function and Regulation== | |
| - | ==Function== | + | PYM |
| - | + | ||
==See Also== | ==See Also== | ||
*[[Prion|Prion]] | *[[Prion|Prion]] | ||
| + | *[http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3haf&template=main.html PDBSum] | ||
| + | |||
==Reference== | ==Reference== | ||
Revision as of 18:52, 8 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||
| 3haf, resolution 2.26Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Gene: | PRNP, PRIP, PRP (Homo sapiens) | ||||||||
| Related: | 3hak | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
A prion is an infectious agent composed of protein in a misfolded form. The name prion, is derived from the words protein and infectious. Prions are responsible for the transmissible spongiform encephalopathies in mammals .In humans, prions cause Creutzfeldt-Jakob Disease(CJD), Fatal Familial Insomnia(FFI) and kuru.
The 3haf structure results from a work leaded by Lee S. in 2010, in which they have characterized seven variants of the human prion. The structure was determined by XRAY diffraction in a 2.26-Angstrom resolution.
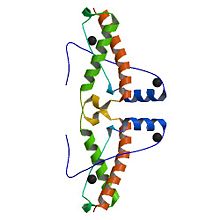
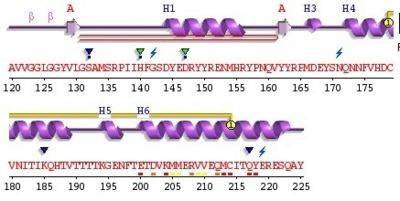
3haf is a domain of the Major prionprotein. This domain is between the residue 120 and the 225 of the entire prion .This structure is a succession of alpha helix and beta sheet. The domain contains sites of glycolisation. It can be noticed a mismatch precisely at the residue 129, where a Valin substitutes a Methionin, influencing the susceptibility of the formation of the prion.
Structure of 3HAF domain of Human Prion
Secondary Structure
The secondary structure is a succession of alpha helix and beta sheet:
Alpha helix (112-135) _ coil_ alpha helix (144-153)_ coil_3/10 helix (165-169)_coil_ alpha helix_ coil_ alpha helix (200-227)
The proportion of each structure is 43% of alpha helix (6 helix, 62 residues) and 2% of beta sheet (2 strands, 4 residues). There are also 2 glycosylation sites and the C-ter domain is higher conserved.
3 residues can have a contact with metals; S132, H140 and D147. The structure shows also an hairpin structure at the N-ter domain. A lot of empty structures (not in the secondary structure) are present between helix. One of this structure is called 3/10 helix (Each amino acid corresponds to a 120° turn in the helix). The entire 3haf domain can interact with Growth factor receptor-bound protein 2 (GRB2), exoribonuclease 3(ERI3) and Synapsin I (SYN1).
In the following representation, we can see the alternationof alpha helix and beta sheet, and the different site of interactions:
Ligand
This domain 3HAF of the human prion can bind copper (II) ions with high affinity: Cd2+ cadnium ions and Cl- (chloride ion). Moreover, the entire protein can bind a Cu2+ ions on this NH2 tail and this bond can induce conformational change with a lot of unknown effect.
In fact, the 3haf domain is only one chain with 2 binding sites for residues CD (H40 and D147)and 1 binding domains for CL S132.
Polymorphism
The common Methionine/Valine polymorphism residue in 129 in the PrP influences the health because a simple mutation on the valine 129 can have an effect on some disease.
If this Valine is muted as methionine (polymorphism) it can determine the disease phenotype in patients. Valine 129 is finding on CJD whereas Methionine 129 is find in FFI.
A lot of others mutations can be finding in these diseases: for example, a substitution of Arginine 208 in Histidine 208 can hae an effect on mental disease and is finding in CJD. At the same way, a subtitution of Asparagine 171 in Serine 171 can be finding in schizoaffective disorder.
For this domain, two glycosylated sites exist on helix 2 and 3 at Asn181 and Asn197. A disulfide bond exist between Cys179 (helix 2) and Cys214 (helix 3).
Biological Application
PYM
Function and Regulation
PYM
See Also
Reference
- Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333